[JAMA] Can PSA screening be “once and for all�
Release date: 2018-03-14
background:
Guidance from all countries around the world on “whether PSA-based prostate cancer screening should be conducted†is not the same. Two clinical trials in Europe (ERSPC, N=162243) and the United States (PLCO, N=76693), although large in sample size, have not resolved this debate. At present, PSA screening is not recommended in the UK; the 2017 US draft guidance recommends that men aged 55-69 decide whether to conduct PSA screening after the doctors fully explore the risks and benefits.
The PSO test was performed on the first, second, and fourth years of the PLCO and ERSPC clinical trials. Tests with weaker intensities, such as longer intervals or even one-time tests, will reduce the frequency of screening for the population, but it is still unclear whether the expected results can be achieved. As a result, a clinical trial of CAP was conducted to evaluate whether there was a difference between the low screening intensity (one-time) PSA screening (intervention group) and the non-screening (control group) prostate cancer mortality rate and all-cause mortality. This article reports the results of this clinical trial at a median follow-up of 10 years.
method:
This study is a stratified cluster randomized trial based on primary health care institutions, with stratification factors being regions. Between 2001 and 2009, a total of 785 eligible primary care institutions in 99 regions were randomly assigned to the intervention group (n=398) and the control group (n=387), and the medical institutions that finally agreed to participate in the study included the intervention group. Of the 271 (68%) and 302 (78%) of the control group. Eligible subjects were invited to perform a PSA test in each institution, and a standardized prostate biopsy was required for subjects with PSA results above 3 ng/mL.
A total of 419,582 men aged 50-69 from 573 primary care facilities in the UK were included in the study, excluding those with a history of prostate cancer, or temporarily registered with the institution. The follow-up deadline is March 31, 2016.
result:
Of the 415,357 subjects randomized, 408,825 (98%) were included in the final analysis, with 189,386 in the intervention group and 219,439 in the control group. Of the 67,713 people (36%) in the intervention group received PSA testing and 64,436 people eventually received valid PSA measurements. A total of 6,857 (11%) of subjects with PSA levels between 3 and 19.9 ng/mL, of which 5,850 (85%) eventually underwent prostate biopsy.
After a median follow-up of 10 years of follow-up, 549 (0.30/1000 person-years) of the intervention group died of prostate cancer, while 647 (0.31/1000 person-years) died of prostate cancer in the control group. The difference in mortality was −0.013 /1000 person-years (95% CI, −0.047 to 0.022), and the relative risk RR was 0.96 (95% CI, 0.85 to 1.08, P = 0.50) (Figure A). The number of patients diagnosed with prostate cancer was higher in the intervention group than in the control group (4.3% vs. 3.6%, RR = 1.19, 95% CI 1.14 to 1.25, P < 0.001) (panel B). The main reason was that the Gleason score was less than or equal to 6 points. The intervention group identified more patients with prostate cancer (1.7% vs. 1.1%, the difference was 6.11, 95% CI, 5.38 ~ 6.84, P < 0.001). . In the total cause of death analysis, the number of deaths in the intervention group and the control group were 25,459 and 28,306, respectively, and the RR was 0.93 (95% CI, 0.67 to 1.29, P = 0.66).
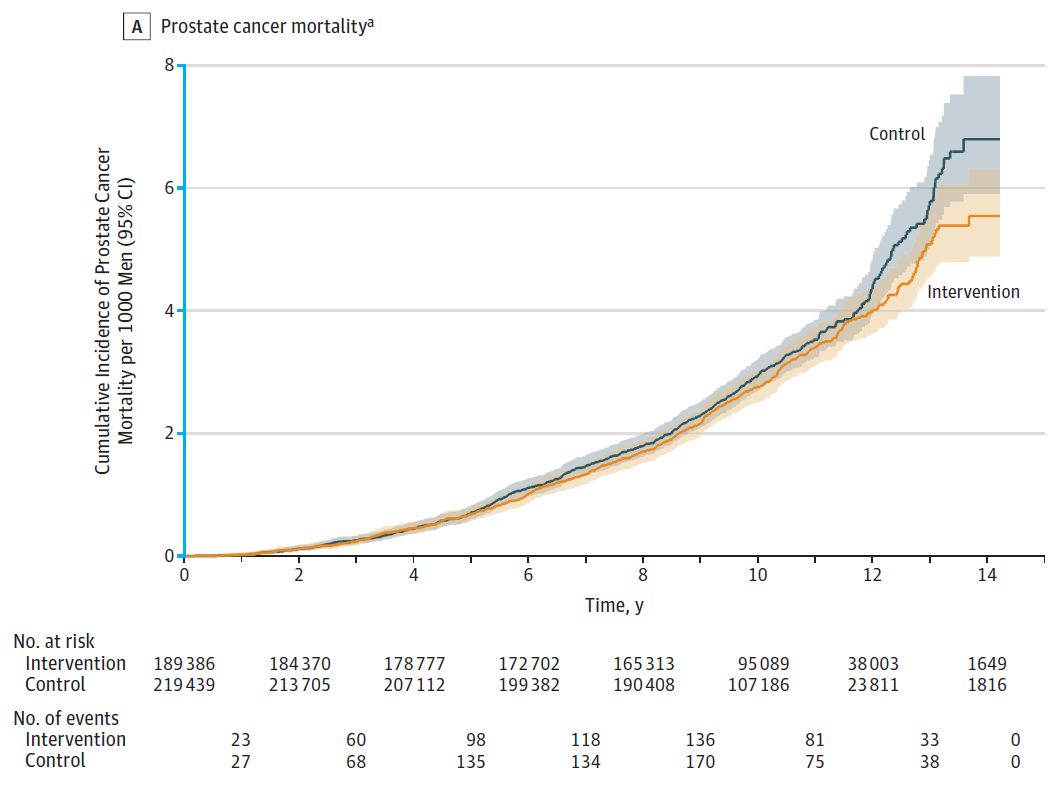
Figure A Comparison of cumulative mortality from prostate cancer per 1000 people in the two groups (median follow-up time 10.03 years)
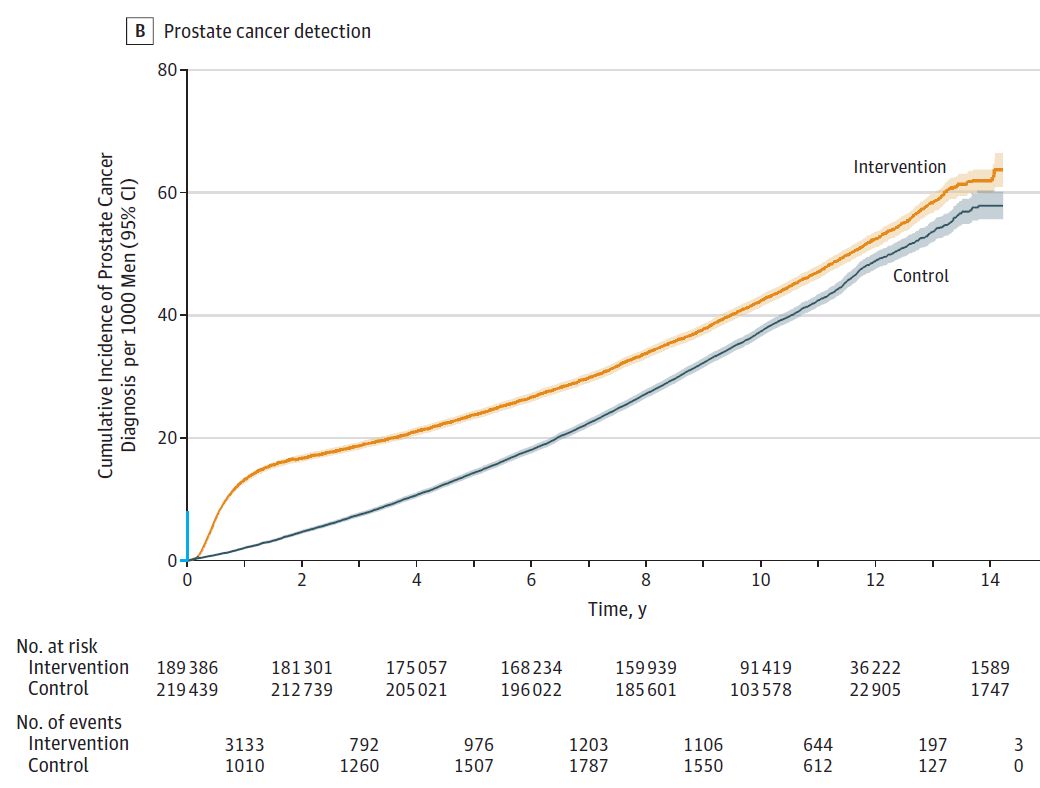
Figure B Comparison of cumulative detection rates per 1000 populations in the two groups (median follow-up time 9.82 years)
in conclusion:
After a 10-year follow-up, there was no significant difference between the population who underwent PSA screening in the intervention population and those who did not. However, low-risk prostate cancer was evident in the screening population. Higher than the population for screening. Although longer follow-up is still ongoing, the current results are not sufficient to support a single PSA screening in the population.
discuss:
The main difference between the two studies of CAP and European ERSPC and American PLCO is that CAP only performed one PSA test (low intensity) on the subject, while ERSPC and PCLO performed multiple tests (high intensity). PSA is a relatively sensitive prognostic indicator for low-risk prostate cancer (Gleason score less than 6 points), so a single PSA test can easily identify many low-risk prostate cancer patients (usually no diagnosis or treatment is required), but at the same time Missed some high-risk patients.
Although the CAP study will continue to follow up for at least 5 years to determine if a single PSA screening can survive for a longer period of time, the current results suggest that screening for prostate cancer should not be based solely on The results of a PSA screening were judged, screening was performed every 2 to 4 years, and multiple screenings showed a sustained increase in PSA, which may be more meaningful for screening for prostate cancer patients.
references
Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA. 2018 Mar 6;319(9):883-895. doi: 10.1001/jama.2018.0154.
Source: Tumor Information
Characteristics disease virus antibody test kit Wondfo Antibody Rapid Test Kit No cross reaction with common respiratory viruses Rich sample type suitable for medical institutions Easy to operate safe
Travel Testing Kit
NINGBO AUTRENDS INTERNATIONAL TRADE CO., LTD , https://www.metests.com