On December 24, 2014, the State Food and Drug Administration issued the Notice on Soliciting the Opinions on the Classification of Medical Devices (Revised Draft for Soliciting Opinions) on its official website. Since 2000, the "Medical Device Classification Rules" has undergone major revisions for the first time. Based on the original "Active Medical Devices" and "Passive Medical Devices", "In vitro Diagnostic Reagents" have been added, and related descriptions have been added. content.
The in vitro diagnostic reagent registration management method (Trial) issued in 2007 stipulates that in vitro diagnostic reagents refer to in vitro diagnostic reagents managed by medical devices, including those that can be used alone or in combination with instruments, appliances, equipment or systems. Reagents, kits, and calibrations for in vitro detection of human samples (various body fluids, cells, tissue samples, etc.) during disease prevention, diagnosis, treatment monitoring, prognosis observation, health status evaluation, and hereditary disease prediction Products (materials), quality control products (objects), etc.
The diagnostic reagents in China are mainly three kinds of biochemical, immunological and molecular diagnostic reagents. Companies involved in the production of diagnostic reagents include Daan Gene, Kehua Bio, Lidman and other manufacturers, accounting for about 40% of the domestic market share.
At present, there are many standards related to in vitro diagnostic reagents, especially for the basic medium used in large quantities, pure water, which has detailed requirements. Purified water (or water for injection) is an integral part of in vitro diagnostic reagents. It involves the steps of production, quality inspection, and use. It is critical to meet the water requirements. The following is a list of relevant regulations related to water use in some standards:
The Regulations for the Production of In Vitro Diagnostic Reagents (Trial) 2007 edition:
Article 25 water equipment process water shall meet the quality requirements and verified, preparation, storage, delivery should be able to prevent microbial contamination and breed not respond affect product quality and performance. Water equipment should be cleaned, disinfected and maintained regularly. Equipment and equipment for water quality monitoring should be equipped and the monitoring results should be recorded regularly.
Article 33 The manufacturer shall establish at least, implement the following basic procedures and record keeping, and supplemented in accordance with the specific requirements of the product:
......
11. Process water regulations and records;
......
Article 57 An enterprise shall formulate procedures for process water use. Verify and specify the quality standards, inspection cycles and shelf life of process water. The corresponding storage conditions and water quality monitoring equipment should be equipped to record and save the monitoring results on a regular basis.
"Good Manufacturing Practice" 2011 Section VI pharmaceutical water requirements:
Article 96 Pharmaceutical water should be suitable for its use and meet the quality standards and related requirements of the Pharmacopoeia of the People's Republic of China. At least drinking water should be used for pharmaceutical water.
Article 97 The design, installation, operation and maintenance of water treatment equipment and its conveying system shall ensure that the pharmaceutical water reaches the set quality standards. Water treatment equipment must not operate beyond its design capabilities.
Article 98 The materials used in purified water, water injection tanks and pipelines shall be non-toxic and corrosion-resistant; the vents of tanks shall be fitted with hydrophobic sterilizing filters that do not shed fibers; the design and installation of pipelines shall avoid dead ends, Blind tube.
Article 99 The preparation, storage and distribution of purified water and water for injection shall be able to prevent the growth of microorganisms. The purified water can be recycled, and the water for injection can be kept at a temperature of 70 ° C or higher.
Article 100 The water quality of pharmaceutical water and raw water shall be regularly monitored and recorded accordingly.
Article 101 The purified water and water for injection pipes shall be cleaned and disinfected in accordance with the operating procedures, and relevant records shall be made. It is found that microbial contamination of pharmaceutical water should be handled in accordance with the operating procedures when it reaches the warning limit and the correction limit.
The "Quality Management System Specification for Medical Device Manufacturing Enterprises" (draft) requires:
The production enterprise shall determine the required process water. When the process water is used in the product realization process, the corresponding water-making equipment shall be equipped and transported to the clean area through the pipeline; the sterile medical equipment in contact with the blood or liquid medicine, the final road Washing water should meet the requirements for water for injection.
The production enterprise shall formulate management documents for process water, and the storage tanks and pipelines for process water shall meet the product requirements and be regularly cleaned and disinfected.
In summary, the in vitro diagnostic reagents have the following requirements for water:
1. Process water must meet Pharmacopoeia requirements (see table below).
| purified water | Water for Injection | Sterilized water for injection |
pH value   | | 5.0 - 7.0 | 5.0 - 7.0 |
Conductivity ( 25 ° C ) | ≤ 5.1 μS·cm-1 | ≤ 1.3 μS·cm-1 | Labeled capacity ≤ 10ml, conductivity ≤ 25 μS·cm - 1 Labeled capacity >10ml, conductivity ≤5 μS·cm-1 |
Total organic carbon | ≤ 0.50 mg/L | ≤ 0.50 mg/L | ≤ 0.50 mg/L |
microorganism | After treatment by membrane filtration, no more than 100 per ml | After treatment by membrane filtration, no more than 100 per ml | No bacteria may be detected according to the sterility test method. |
2. Must pass verification.
3. Storage and transportation must be guaranteed.
In order to meet the demand for water for in vitro diagnostic reagents, RephiLe can provide users with a reliable and pure water preparation system. The Direct-Pure series water purification system produced by RephiLe uses tap water as the influent water. According to the user's requirements, it can produce secondary reverse osmosis RO water or EDI pure water. The water conductivity and total organic carbon meet the 2010 Pharmacopoeia for purified water. Conductivity requirements for water for injection, etc. The pipes and storage tanks are made of 316L stainless steel. The storage tank is equipped with a hydrophobic membrane sterilization filter and can provide a constant temperature of 80 °C, which meets the requirements for storage of purified water and water for injection. RephiLe also offers a full suite of validation procedures for water purification systems, with proven engineers, proven instruments, and validated workbooks to assist users with system validation such as GMP. In addition, RephiLe also offers the PURIST ultrapure water system, which uses purified water as feed water to produce ultrapure water. The water production meets the first-class water requirements of the Analytical Laboratory Water Specifications and Test Methods (GB6682-2008), meeting the requirements of the quality inspection laboratory of the manufacturer of the in vitro diagnostic reagents. At the same time, RephiLe provides users with perfect and professional after-sales service, so that users can worry freely, worry-free and worry-free.
Longpass Filter
An optical coating that uses the principle of interference to pass only light in a specific spectral range. It is usually composed of a multilayer film. There are many types of interference filters with different uses. Common interference filters are divided into cut-off filters and band-pass filters. The cut-off filter can divide the spectral range into two regions, where the light in one zone cannot pass (cut-off zone), and the light in the other zone can fully pass (pass-band zone). Typical cut-off filters include low-pass filters (allowing only short-wave light to pass through) and high-pass filters (allowing only long-wave light to pass through), both of which are multilayer dielectric films with a high refractive index layer and a low refractive index. Periodic structure composed of alternating layers. For example, the simplest high-pass filter structure is g(L/2)(HL)mH(L/2)a, where g stands for glass (optical element material), a stands for air outside the film, and L and H stand for The low refractive index layer and the high refractive index layer with a thickness of 1/4 wavelength, L/2 represents the low refractive index layer with a thickness of 1/8 wavelength, and m is the number of cycles. Similarly, the junction of the low-pass filter is g(H/2)L(HL)(H/2)a. The structure of a high-pass and low-pass filter with a symmetrical periodic film system is g(0.5LH0.5L)ma and g(0.5HL0.5H))ma respectively.
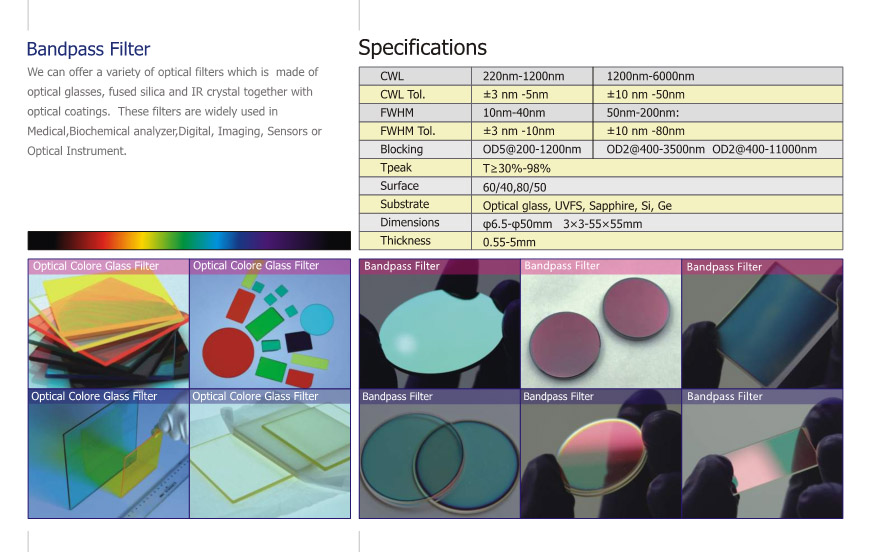
Longpass Filter,Long Pass Filter,Optical Longpass Filter,Custom Longpass Filter
ChangChun Worldhawk Optics Co.,Ltd , https://www.worldhawk-optics.com
