Pre-freezing is to solidify the free water in the solution, giving the dry product the same form as before drying, preventing irreversible changes such as foaming, concentration and solute movement during evacuation and drying, and minimizing the solubility reduction and life characteristics of the substance caused by temperature. The change.
1 , the method of pre-freezing
There are two pre-freezing methods for the solution: pre-freezing in the freeze-drying box and pre-freezing in the box.
The pre-freezing method in the box is to directly place the product on the multi-layer shelf in the lyophilizer, and freeze it by the freezer of the lyophilizer. When a large number of vials and ampoules are lyophilized, it is convenient for entering and unpacking. Generally, the vials or ampoules are placed in several metal trays and then placed in the box in order to improve heat transfer. Some metal discs can be made into a movable bottom type. When entering the box, the bottom is pumped away, so that the vial is directly in contact with the metal plate of the freeze-drying box; for the non-priming plate, the bottom of the plate is required to be flat to obtain uniformity of the product. The large plasma bottle using the spin-drying method should be pre-frozen and then added to the metal frame for heat conduction and then into the box for freezing.
There are two methods for pre-freezing outside the box. Some small freeze dryers do not have a pre-frozen product and can only be pre-frozen using a low temperature refrigerator or alcohol plus dry ice. The other is a special type of chiller that freezes the product of the large bottle into a shell-like structure and then into the lyophilization box.
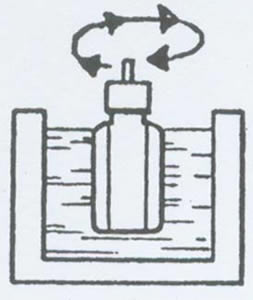
Figure 1 Rotary Freezing
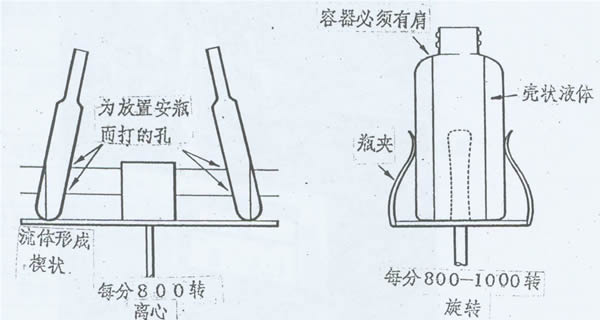
There is also a special centrifugal pre-freezing method, which is used in centrifugal lyophilizers. The liquid is quickly evaporated by vacuum and absorbed by the heat itself to freeze. The centrifugal force of rotation prevents the product from escaping, allowing the product to freeze "quietly" into a certain shape. The rotational speed is generally around 800 rpm.
Figure 2: Forming a wedge or shell by centrifuging or rotating the liquid
2 , the process of pre-freezing
When the temperature of the aqueous solution drops to a certain level, according to the eutectic concentration of the solution, the concentration begins to freeze in the light solution. This temperature is called the freezing point. Generally, the freezing point is controlled by the concentration and the concentration is lowered. When the temperature of the solution is lower than the freezing point, a part of the solution will crystallize, and the concentration of the remaining solution will rise, so that the freezing point will fall, and then continue to cool, and the ice crystal will increase with cooling, and the remaining solution concentration It increases with it. However, when the temperature drops to a certain point, the remaining solution is completely frozen. At this time, the frozen matter is mixed with ice crystals, and the temperature at this time is the eutectic point.
After the solution needs to be cooled to the freezing point, after the nucleus is generated, the free water will begin to crystallize in the form of ice, and the heat of crystallization will be released to raise the temperature to the freezing point. As the crystal grows, the concentration of the solution increases. When the eutectic concentration is reached and the temperature drops below the eutectic point, the solution will all freeze.
The number and size of crystal grains crystallized by the solution are related to the nucleation rate and crystal growth rate in addition to the nature of the solution itself. The two factors, nucleation rate and crystal growth rate, change with temperature and pressure. Therefore, we can control the temperature and pressure of the solution to control the number and size of crystal grains in the solution. In general, the faster the cooling rate, the lower the subcooling temperature, the more the number of crystal nuclei formed, the more the crystals are frozen before they grow, and the more crystal grains are formed, the finer the crystal grains; The smaller the number, the larger the grain size.
The shape of the crystal is also related to the freezing temperature. When freezing starts around 0 °C, the ice crystals are hexagonal symmetrical and grow forward in the direction of the six major axes. At the same time, several secondary axes appear, and all the ice crystals are connected to form a network structure in the solution. As the degree of subcooling increases, the ice crystals will gradually lose the hexagonal symmetry form of capacity recognition. In addition, the number of nucleation is large, and the freezing speed is fast, which may form an irregular branch type, and they have any number of axial columns. Not as many as six hexagonal crystals.
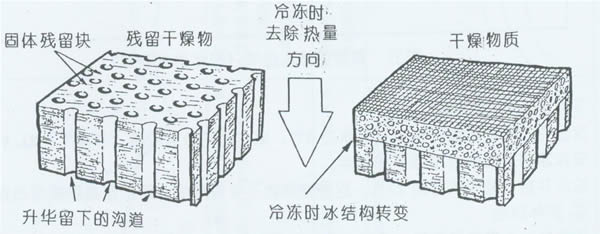
Figure 3 Freeze-dried shards, indicating voids after sublimation (no proportion)
Crystallized units formed by freezing of biological fluids (such as blood plasma, muscle serum, vitreous humor, etc.) tend to be similar to the type of ice crystal formed by a single component aqueous solution. The type of crystallization mainly depends on the cooling rate and the concentration of body fluid. For example, plasma, muscle slurry, etc., when frozen at normal concentration, form hexagonal crystal units at higher subzero temperatures and slow cooling rates, and form irregular dendrites when rapidly cooled to low temperatures. Crystal.
Cell suspensions (such as red blood cells, white blood cells, sperm, bacteria, etc. suspended in distilled water, plasma or other suspension medium), when the temperature is slowly frozen at high temperatures, a large amount of ice grows in the suspension, and the cells are squeezed into two icicles. In the narrow pipeline, the suspended medium in the pipeline is concentrated by the precipitation of water and the solute is concentrated, and the water in the cell permeates out of the cell through the cell membrane, which causes concentration of the intracellular solute. At the same time, the growth of extracellular ice will also force the volume of the cell to shrink and deform. But at this time, there is no ice in the cells. When it freezes rapidly at low temperatures, intracellular ice will form inside the cells. The size, shape and distribution of ice are related to the cooling rate, the presence or absence of a protective agent, the nature of the protective agent, and the amount of water in the cells. Generally speaking, the faster the cooling rate and the lower the temperature, the more ice is formed in the cells. . The addition of a non-permeable protective agent to the suspension reduces the amount of ice formed in the cells during rapid freezing.
Â
The form of solution crystallization has a direct effect on the lyophilization rate. The gap left after sublimation of the ice crystal is the escape passage of water vapor after the subsequent ice crystal sublimation. The large and continuous hexagonal crystal sublimation forms a large gap channel, and the resistance of water vapor escape is small, so the product drying speed is fast, and vice versa. The discontinuous spherical ice crystal channel is small or discontinuous, and the water vapor can escape by diffusion or permeation, so the drying speed is slow. Therefore, only from the drying rate, slow freezing is good.
In addition, the rate of freezing is also related to the type of freezing equipment, capacity, and heat transfer medium.
Pre-freezing will have a certain destructive effect on cells and life. The mechanism is very complicated. It is generally believed that the mechanical and solute effects of water freezing during pre-freezing process cause biochemical drugs to be inactivated during lyophilization or An important factor in degeneration. The mechanical effect refers to an increase in volume when water freezes, causing some weak molecular bonds in the active site of the active material to be destroyed, thereby causing loss of activity; the solute effect refers to an increase in solute concentration after water freezing and due to various solutes in each Inconsistent changes in solubility under temperature conditions cause changes in pH, resulting in changes in the environment in which the active substance is exposed, resulting in deactivation or denaturation. The following measures can be taken to solve this phenomenon: 1 Pre-freezing adopts the quick freezing method, firstly reduces the shelf temperature to -45 °C, and then puts the product into rapid freezing to form fine ice crystals, which makes it too late to produce mechanical effects. 2 When using a buffer, a buffered paired salt with a similar solubility should be used. 3 Add product protection agent.
Handheld Laser Distance Meter
JRT OEM Digital Laser Range Finder can measure faster, easier, and more effectively! Our Professional R&D team has over 16 years of experience on the laser and measurement field , so we can supply perfect OEM/ODM service according to user requirement ,and we have made out the mini size module in the world.
The handheld laser distance measurer can measure distance,area,volume and pythagorean, and can switch to meter/feet/inch mode as you like.
Specially, there is three best-sale styles:
* Mini: D30 with our mini module U81 in it achieved the smallest size Laser Distance Meter in the world, which outlook is the same as a key,quite portable and cute. USB chargable design.
* Bluetooth: Diy Laser Range Finder With Bluetooth has been used to measure distances accurately in the both daily usage and industry application. The handheld laser distance measurer is widely used in variety areas, like project surveying, construction measuring, railway application etc.
* Longest: 150m equipped with a precise,easy- to-read digital LED display, which metal case brings you great hand feel.Power Supply: 2 x 1.5V AAA batteries.
Handheld Laser Meter,Handheld Laser Distance Meter,Laser Range Meter,Laser Distance Measurer
Chengdu JRT Meter Technology Co., Ltd , https://www.irdistancesensor.com