How to go for the commercialization of high-cost gene therapy?
Release date: 2017-09-11
On Wednesday, local time, the FDA officially approved the Novartis CAR-T therapy Kymriah (formerly known as CTL-019) for the treatment of relapsed or refractory (r/r) children and young adult B-cell acute lymphoblastic leukemia ( ALL). This is the world's first approved CAR-T therapy and the first gene therapy approved by the FDA.
Previously, two gene therapy products were marketed in Western countries, namely Glybera from UniQure and Strimvelis from GSK, both of which are used to treat ultra-rare diseases. However, due to high expenses, medical insurance access problems and a small patient base, the sales of these two products are bleak. UniQure intends to delist Glybera and GSK plans to sell the Rare Diseases business unit. At present, there are many layouts in the field of gene therapy, but the commercialization of such products needs to be solved urgently.
Back at Kymriah, Novartis set its price as a treatment of $475,000 for the cost of general concern. Novartis said it will work with the US Medicare and Medicaid Services Center (CMS) to pay for it based on efficacy. Only when the patient responds to the therapy by the end of the first month of treatment, Novartis can receive payment from the CMS.
The process of receiving Kymriah treatment is very different from the general medicine. Patients need to go to the treatment center set up by Novartis in the United States. The doctor will collect the white blood cells of the patient and send it to the factory for processing and then return it. The whole process takes about 22 days. Kymriah's $475,000 treatment fee does not include travel expenses for patients, hospitalization, and other medications for Kymriah side effects.
Novartis executives revealed that at the beginning of Kymriah's listing, there will be 20 treatment centers in operation, which will increase to 32 by the end of the year. In the next 3 to 5 days, some treatment centers will begin to collect patients' T cells.
How big a market Kymriah can create is not yet known. In the United States, there are about 3,100 new ALL patients each year, but about 70% of them have been treated with conventional treatment to alleviate the condition. How much can Novartry's huge investment generate? The outside world has doubts about this.
Of course, the potential of Kymriah has yet to be tapped more widely. Novartis is investigating its use in the treatment of lymphoma. In addition, other CAR-T therapies on the company's research and development line are also conducting research on a range of blood cancers. Kite Pharmaceuticals, which was acquired by Gilead, is still awaiting FDA's review of its CAR-T products. Like Novartis, cell therapy developed by Kite Pharmaceuticals is also intended to cover the treatment of solid and non-solid tumors.
Link>>>
Gene therapy development is hot
There are many layouts in the field of gene therapy, but what needs to be solved is the commercialization of such products.
Thanks to advances in gene delivery technology targeting targeted cells, more and more gene therapy products are on the market to correct DNA in patients with defects.
In Europe, the first two gene therapy products, Glybera and Strimvelis, are used to treat ultra-rare diseases, and their unsatisfactory sales reflect the marketization of such extremely expensive innovative products.
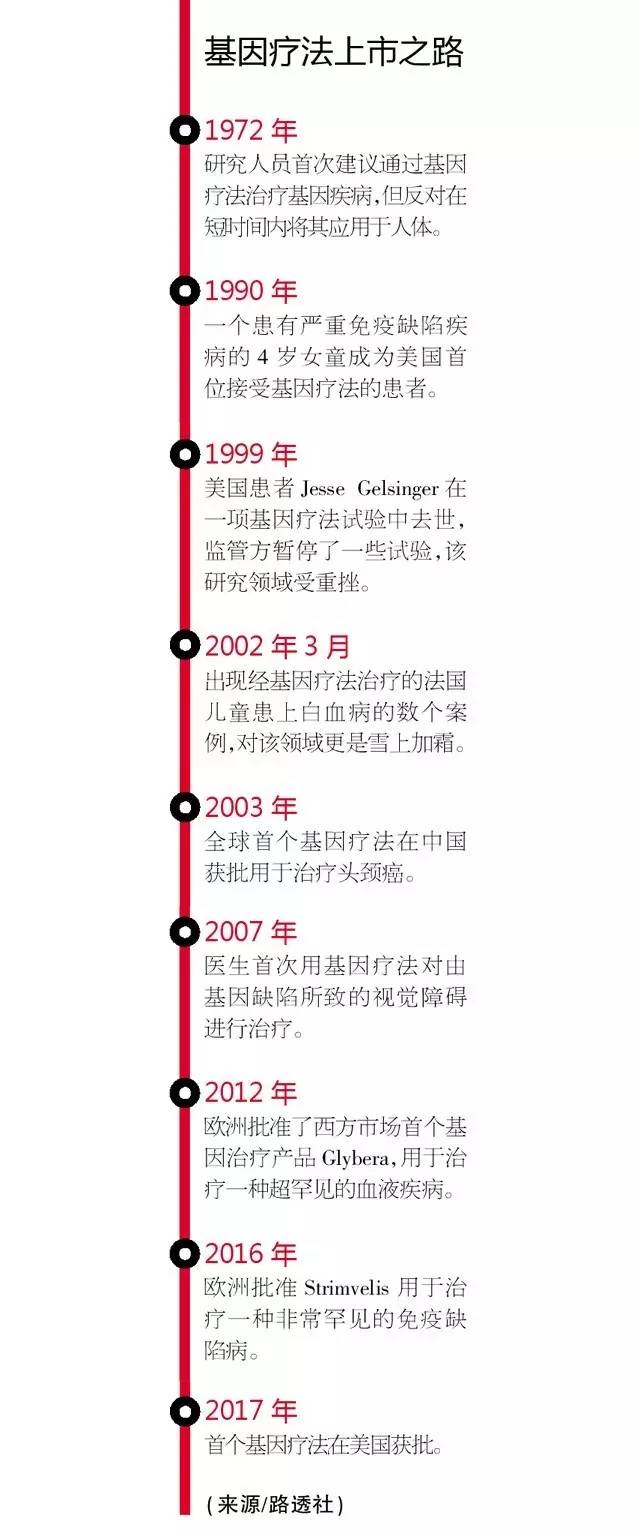
2 listed products sold bleak
In the 1990s, research using genetically engineered viruses to transport normal genes was very hot. However, such studies have been hit hard by the death of one patient after treatment and the cancer in several patients after treatment. However, the safety of the virus has been greatly improved and the transshipment efficiency has been greatly improved.
After decades of ups and downs, pharmaceutical companies believe that they are now a great opportunity to develop gene therapy products for hereditary diseases such as hemophilia. They believe that gene therapy can cure these diseases at one time. In the long run, gene therapy can save more money for health care institutions than lifelong treatment using current routines.
In the past five years, the European Medicines Agency (EMA) has approved two gene therapy products, but only three patients have received treatment so far.
UniQure's product, Glybera, is used to treat rare lipoprotein lipase-deficient genetic diseases, and UniQure intends to delist it due to lack of market demand. GlaxoSmithKline is used to treat ADA-SCID (severe combined immunodeficiency caused by adenosine deaminase deficiency, which is a glass child, and patients are very susceptible to infection due to immunodeficiency). Strimvelis has an uncertain future because the company Deciding to adjust its R&D department for rare diseases, it is very likely that this sector will be sold.
Glybera is priced at about $1 million and has only sold one since its launch in 2012; Strimvelis is priced at about $700,000, and only sold two in May 2016, and two will be available later this year. Patients scheduled to receive treatment.
Hilary Thoma, KPMG's Chief Medical Consultant, noted that the disappointing number of people receiving gene therapy in Europe shows the commercial challenge of developing these products and highlights the government's health care system paying for such disposable, expensive therapeutic products. There are problems on it.
These two products are aimed at extremely rare diseases. GSK estimates that there are only 15 new ADA-SCID patients in Europe each year, but these two products lay the foundation for the later development of more common hereditary disease gene therapy.
Payment methods must be reformed
In the United States, gene therapy products are also accelerating the pace of listing: Novartis's CAR-T product, Kymriah, has been approved, and Spark hopes to list its gene therapy products for hereditary blindness in January next year.
At the same time, scientific research institutions have made significant breakthroughs in the development of gene therapy. Recently, researchers successfully used the CRISPR-Cas9 gene editing technology to genetically correct human-deficient embryos, which is a more innovative therapy.
Spark CEO Jeffrey Marrazzo has his own opinion on the bleak sales of the first batch of gene therapy products in Europe. He believes that the reasons include the complexity of the medical insurance system, the flaws in Glybera's clinical trials, and the limitations of Strimvelis's sales in a single hospital in Italy. Sex.
Marrazzo expects Spark to do even better. It plans to set up treatment centers in different countries. The company's products target about 6,000 patients with blindness worldwide. Marrazzo admits that it is a lot of difficulty to recover the company's $400 million invested in the product, because the current medical insurance system is based on monthly medical bills and is not suitable for a one-time payment of gene therapy products.
For a one-time treatment, even if the cost is as high as $1 million, it is cost-effective in the long run. For example, kidney transplantation can save huge medical expenses for long-term dialysis.
However, companies that focus on gene therapy development, such as Bluebird Bio, BioMarin, Sangamo, and GenSight, may need new business models.
One of these models is a payment system based on efficacy, in which the government or insurance company will continue to pay for the product until it has no therapeutic effect. Marrazzo believes that this method is especially suitable for genetic diseases such as hemophilia, which can significantly save on treatment costs.
It is estimated that there are more than 100,000 hemophilia patients, and currently some specialist pharmaceutical companies are in control of the treatment market. Hemophilia may be the most promising market for gene therapy. After receiving gene therapy, patients can get rid of the long-term infusion of blood clotting drugs. Current conventional treatment costs up to $400,000 per year.
It is worth mentioning that although GSK no longer develops gene therapy products for rare diseases, it is still studying the use of gene therapy technology platforms to develop common disease therapies, including tumors and thalassemia. Thalassemia is also a hereditary blood disease.
Older competitors such as Pfizer and Sanofi have also developed the development of gene therapy products. According to the statistics of the Regenerative Medicine Alliance, the cumulative investment in gene therapy products and genetically modified cell products in the first quarter of this year alone One billion dollars.
Shire CEO Flemming Ornskov believes that the opportunities and difficulties of gene therapy coexist. Shire has a large market share in the field of conventional therapy for hemophilia, and is following Biomarin and Spark in the development of gene therapy products for hemophilia. “If the clinical research data is still encouraging, gene therapy products will have a certain market share in the medium and long term. But I think that each company must have its own business model to ensure the profit of the product.†Anskov pointed out .
Source: Pharmaceutical Economics
Enhance Memory Ingredients,Natural Phosphatidylserine,Anti-Oxidation Phosphatidylserine Powder,Soybean Extract Phosphatidylserine Powder
Xi'an XJeson Biotech Co., Ltd , https://www.xjesonbio.com