Application of Plant-derived Recombinant Human Serum Albumin in Serum-free Culture of Cell Therapeutic Products
Cell therapy technology is currently the key development field of international medical frontiers, which provides new hopes for the treatment of some human difficult diseases. In recent years, new research results have been continuously obtained in the field of cell therapy. Among them, Car-T therapy has become increasingly hot in recent years and the competition is fierce. Up to now, there are two Car-T products approved worldwide: Kymriah of Novartis and Yescarta of Kite Pharmaceuticals, while in China, Nanjing Legend, Koji Bio, Galaxy Bio, Shanghai Hengrun Dasheng, Bosheng Ji'anke and Ming The Car-T therapy of Polybio has also been accepted by the State Food and Drug Administration and entered the clinical research stage.
However, the research and development specifications, safety evaluation, effectiveness and quality controllability of cell therapy products need to be further regulated. Based on this, on December 22, 2017, the State Food and Drug Administration (CFDA) issued "Technical Guidelines for Research and Evaluation of Cellular Therapeutic Products (Trial)", hereinafter referred to as technical guidelines.
Among them, whether the serum can be used in the cell culture process for the safety of the production process, the technical guidelines clearly indicate: “Serve serum from any source, including human serum, should be avoided as much as possible. If necessary, the applicant should provide sufficient research data. Explain the necessity of using human serum in the cell culture process. At the same time, the value of the clinical application of the product will be evaluated in terms of risk and benefit during the technical review process. Under the precursor that meets the necessity of human serum use, apply The person needs to provide the selection basis of the human serum used, the safety research data and the applicant's control strategy for human serum quality, etc., and the serum that has not been verified by safety should not be used in the cell treatment products. With the development and innovation of technology, Applicants are encouraged to actively explore safer, more defined serum replacements for subsequent research and production."
Since the development of low serum/serum-free culture technology, human serum albumin has been widely used in the research and application of serum replacement. The main functions of human serum albumin in cell culture are mainly divided into:
- Maintain osmotic pressure and pH of the culture system
- Binding and transport of important ligands such as lipids, metal ions, amino acids and other factors
- Antioxidant function
A large number of studies have shown that the addition of human serum albumin in serum-free medium can achieve stable growth after gradually acclimating and adapting cells, and even synergistically promote other cell growth factors to enhance cell growth.
However, most of the commercially available human serum albumin is derived from natural human blood extraction and purification, and may contain viruses and prions, and there are unsafe risks such as the risk of infection such as AIDS or liver diseases. In addition, human serum albumin belongs to the clinical shortage of medicine products. At present, domestic enterprises can only meet 1/4 of the production, and 2/4 of foreign imports each year, there is still a 1/4 shortage. And with the recent increase in blood supply, the production capacity of natural human serum albumin is difficult to meet the large-scale production and supply of biomedical raw materials in the case of shortage of clinical supply of medicine.
Since the 1990s, the state has begun to incorporate human serum albumin into national key strategic projects. Wuhan Heyuan Biotechnology Co., Ltd. (hereinafter referred to as “Woyuan Bioâ€) is in the national “863†program, “a major special project for the creation of new drugsâ€. Supported by the “GMO Major Project†and “National Strategic Emerging Industriesâ€, the recombinant human serum albumin was recombined in the plant system using the rice endosperm cell expression technology platform. Compared with natural human serum albumin, there is no animal source component. There is no risk of any viral infection, and it is possible to achieve super capacity expansion through large-scale planting of rice. And on April 28, 2017, it was approved by the National Food and Drug Evaluation Center for clinical research. Plant-derived recombinant human serum albumin injection is the first one based on rice endosperm cell bioreactor production in China and even in the world. Innovative drugs and clinical approvals are important milestones in recombinant human serum albumin.
In addition to the pharmaceutical market, Heyuan Bio has also developed cell culture-grade recombinant human serum albumin for cell culture, which is dedicated to biopharmaceuticals, cell therapy and other biomedical materials, helping major companies to improve their production processes and improve safety. Controllable system. In addition, Heyuan Biological's plant-derived recombinant human serum albumin has achieved annual production of tons of products, all systems have passed ISO certification, in line with GMP production standards, and are widely used in serum-free culture by well-known cell culture companies at home and abroad.
The main ways to understand the specific information of recombinant human serum albumin from Heyuan biological plant source are:
Welcome to call
Add technical support QQ
Concerned about Woyuan Biological official WeChat public number: oryzogen
Log in to Woyuan Bio official website:
Single Packed Yellow Waxy Corn Cob
For the most common waxy and sweet corn on the market, waxy corn has a higher nutrient content than regular corn, containing 70-75% starch (and almost all straight-chain starch), more than 10% protein, 4-5% fat and 2% multivitamins, with more grains, VA, VB1 and VB2 in protein than rice, with the highest fat and VB2 content. Yellow maize also contains carotenoids, such as rice and wheat. The molecular weight of waxy maize starch is more than 10 times smaller than that of ordinary maize, and the starch makes glutinous rice sticky and soft, softer than ordinary hard maize. It is more than 20% more digestible to eat than regular maize and it is suitable for people with less than perfect teeth. At the same time, it is not suitable for diabetics because of the very high content of straight-chain starch (a polysaccharide).
Waxy maize is also known as sticky maize. The grain has coarse, waxy endosperm, similar to shiny, glassy (clear) grains such as hard and dent maize. Its chemical and physical characteristics are controlled by a recessive gene, which is located on chromosome 9. 100% of the starch in the endosperm is straight-chain starch.
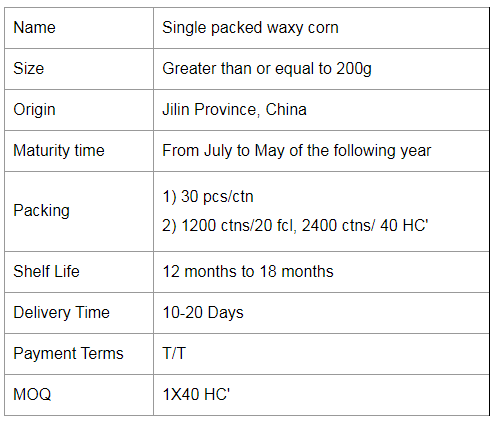


If you have any further questions about our products, please do not hesitate to contact us.
Waxy Corn Cob,Yellow Glutinous Corn,Yellow Waxy Corn Cob,Single Packed Yellow Waxy Corn Cob
Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nongsaocorns.com