Welcome to break through! This artificial protein designed by Great God will be deformed.
Welcome to break through! This artificial protein designed by Great God will be deformed.
May 20, 2019 Source: Academic Jingwei
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];When it comes to artificially designed proteins, Professor David Baker of the University of Washington is the "big god" in the industry. In the calculation of simulated protein function and manual design, the team led by the 50-year-old American Academy of Sciences represents the frontier of the field. Earlier this year, his team first designed an anti-cancer protein from scratch, and the results were also published in Nature.

â–² Professor David Baker is one of the leading figures in the field of artificial design proteins (Source: HHMI)
Last week, in the top academic journal Science, Baker's team once again brought new breakthroughs. This time, the protein they designed will be deformed!
Is the protein "deformed"?
Can this study be published in Science? This is to start with its meaning. With the help of computers, we have obtained a series of principles of protein design, and can design the amino acid sequence with the lowest free energy from the beginning, so that the three-dimensional protein structure formed by it has high stability.
This is certainly a major breakthrough in artificially designed proteins, but it is significantly limited in practicality. These artificial proteins are too stable, just as hard as "rock." They can be good structural frameworks or can bind tightly to other proteins, but in nature, many natural proteins are more flexible and can exhibit different conformations in different environments. These proteins also tend to have a "switch" function that uses different conformations to do different things.
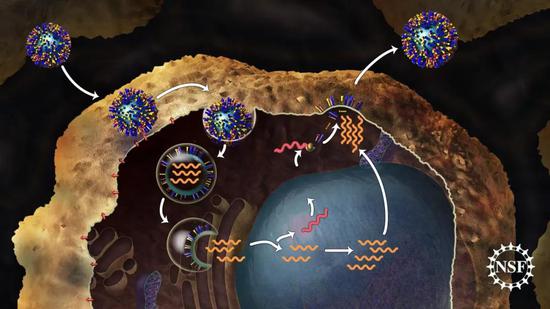
â–² "Deformation" of influenza virus hemagglutinin protein is essential for the release of genetic material of influenza virus (Source: NSF)
For example, after the influenza virus is endocytosed into cells, the surface hemagglutinin (HA) protein will "deform" in an acidic environment, causing the cell membrane of the endosome to fuse with the lipid membrane of the influenza virus. So that the genetic material of the influenza virus can smoothly enter the cytoplasm and begin to replicate.
It can be seen that “deformation†can add a lot of extra functions to the protein, and it is also one of the protein characteristics that scientists want to understand and control. But it is easier said than done. The protein molecules that have evolved over billions of years in nature have an amino acid sequence that not only forms the ideal structure, but also changes according to the surrounding environment. How easy is it to crack a natural password?
Full of human wisdom
But the team of Professor Baker did it!
Over the years, the team has accumulated a lot of experience to focus their attention on an amino acid called histidine. Under neutral conditions, this amino acid has no charge. But when the environment becomes slightly acidic, it carries a positive charge, which affects its ability to form hydrogen bonds. The researchers concluded that this property can help the protein "deform".
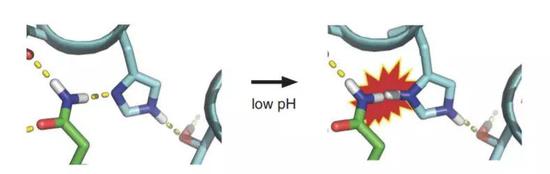
â–²Histidine is the key to protein "deformation" (Source: Reference [1])
Subsequent research also confirmed the reliability of this idea. The researchers used computer simulation to design a synthetic protein. After optimization and purification, it can self-assemble under neutral conditions to become a multimer.
Next, it is the moment to witness the magic. Under acidic conditions, the histidine of these proteins rapidly carries a positive charge, destroying the hydrogen bond network inside the protein, allowing the assembled polymer to begin to disintegrate. This action exposes the protein to hydrophobic residues, making it an amphiphilic molecule. This not only promotes the binding of protein monomers to the lipid membrane, but also affects and destroys the structure of the membrane, allowing the membrane to fuse.
Using cryo-electron microscopy, the researchers confirmed that these proteins can bind to liposomes under acidic conditions. In an alkaline environment, this ability to combine disappears. In addition, the researchers also confirmed that this binding must rely on the presence of histidine. If histidine is replaced with other amino acids, these proteins will not bind to the liposome even under acidic conditions.
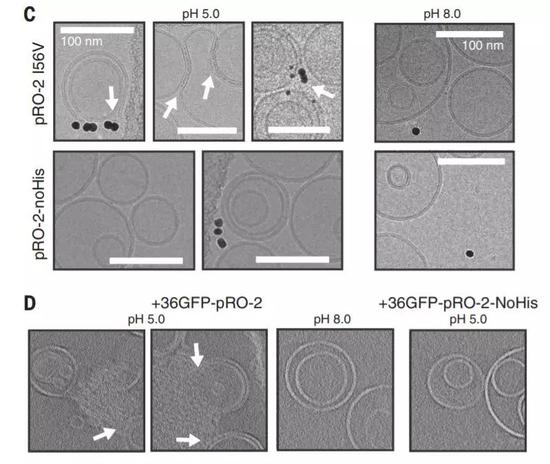
â–² Under acidic conditions, artificially designed proteins can bind and destroy the membrane structure of liposomes (Source: Reference [1])
These results indicate that, through human design, the protein does "deform" under acidic conditions and reveals the desired characteristics that cause the membrane to fuse.
Transforming drug delivery?
In mammalian cell experiments, the "deformation" ability of this artificial protein was once again verified. First, the researchers modified the deformable proteins to give them green fluorescence. They then added these proteins to the cell culture medium.
Subsequent research found that cells would "swallow" them all. Just as people rely on acidic gastric juice to digest food, cells also send endocytic substances to the "lysosome" for digestion. Interestingly, these proteins are highly coincident with the location of the lysosomes, indicating that they have entered the interior of the lysosome. But these proteins still fluoresce, indicating that they have not been digested.
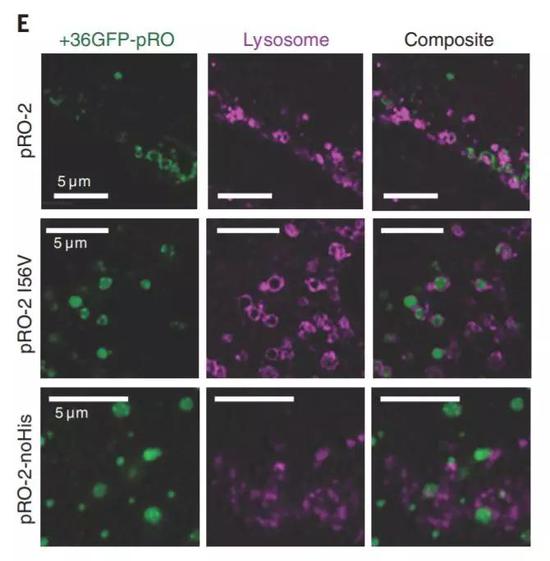
â–²Into the lysosome but not degraded, highlighting the effect of the "deformation" of these proteins (Source: References [1])
These results clearly illustrate the successful "deformation" of the protein. The researchers explained that after being swallowed by the cells, these proteins begin to deform in the acidic lysosomes, thereby destroying the membrane structure of the lysosomes. This is like a stomach that breaks a hole and lets the stomach acid flow out, which naturally weakens the digestive capacity of the lysosome.
Similarly, if the histidine in these deformable proteins is changed to other amino acids, these proteins lose their ability to deform. In lysosomes, they are digested to be clean.
At the end of the paper, the researchers pointed out that if you want to deliver biologics to the cytoplasm, you need to use a lot of "rough" methods, which may bring toxic side effects. Although viral vectors appear to be less "rude," they can also pose an immune risk. The protein that deforms with pH in this study has the potential to release molecules in the endosome into the cytoplasm, and thus is expected to bring about a novel drug delivery method.
“Designing a protein that can change in a predictable way is expected to bring a new wave of molecular medicine,†Professor Baker said. “These molecules are able to penetrate the endosomes and therefore have the potential to bring new tools for drug delivery.â€
Small Cable Reel,Self Retracting Cable Reel,Auto Rewind Water Hose Reel,Wall Mount Water Hose Reel
NINGBO QIKAI ENVIRONMENTAL TECHNOLOGY CO.,LTD , https://www.water-hose-reel.com