Sihuan Pharmaceutical "spoiled" 5 billion market, that is, matching infusion products were approved for production
Medical Network May 28th, May 27th, Sihuan Pharmaceutical announced that the non-PVC powder double-chamber ceftazidime/sodium chloride injection jointly developed by the company and the affiliated company Beijing Ruiye has been awarded by the State Food and Drug Administration. Drug registration approval. According to the data from the intranet, the sales of ceftazidime in China's public medical institutions in 2017 was 5.351 billion yuan. At present, the domestically approved ceftazidime is a powder injection, and Sihuan Pharmaceutical is the first company to obtain a non-PVC powder double-chamber ceftazidime/sodium chloride injection drug registration approval.
Ceftazidime is a semi-synthetic third-generation cephalosporin antibiotic mainly used for sepsis caused by sensitive Gram-negative bacilli, lower respiratory tract infection, abdominal and biliary infection, complicated urinary tract infection and severe skin and soft tissue infection. It is especially suitable for immunodeficiency infections, nosocomial infections, and central nervous system infections caused by Gram-negative bacilli or Pseudomonas aeruginosa caused by multiple drug-resistant Gram-negative bacilli, and the efficacy is clear.
According to the intranet database, the sales of antibacterial drugs for the whole body of Chinese public medical institutions in 2017 was 156.505 billion yuan, an increase of 2.18% over the same period of last year. The sales amount of cephalosporin antibacterials was 80 billion yuan, accounting for the total amount of antibacterial drugs for systemic use. 51.12% of the market.
Figure 1: Sales of terminal ceftazidime in public medical institutions in China in 2013-2017 (unit: 10,000 yuan)
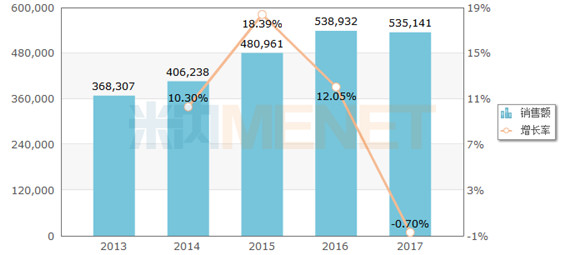
(Source: Minernet China's public medical institutions terminal competition pattern)
In 2017, the sales of terminal ceftazidime in China's public medical institutions was 5.351 billion yuan, accounting for 6.7% of cephalosporin antibacterials. At present, the domestically approved ceftazidime is a powder injection. Sihuan Pharmaceutical is the first company to obtain a non-PVC powder double-chamber ceftazidime/sodium chloride injection drug registration approval. It is expected to form a certain substitute for the existing products. Good market prospects.
Non-PVC powder double-chamber bag, which is equipped with infusion technology, has high technical barriers and long development cycle. It is an internationally advanced infusion product. At present, it is only produced by pharmaceutical companies in the United States and Japan. The dosage form avoids the secondary pollution caused by the dispensing process, eliminates the potential hazard to the medical staff such as the highly sensitizing drugs in the preparation of the infusion process, and has obvious advantages in emergency emergencies or applications in extreme environments. Clinically, it is recognized as the safest, most reliable and most convenient infusion product, and is one of the most promising new formulations in the pharmaceutical industry.
Figure 2: Beijing Ruiye part of the trial variety
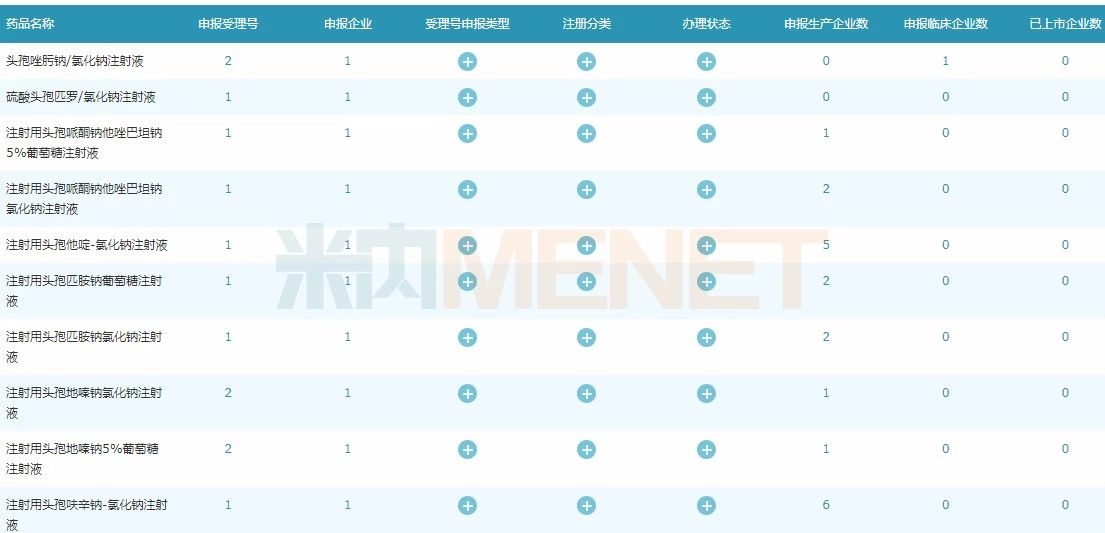
(Source: Minenet MED China Drug Evaluation Database 2.0)
In addition, Beijing Ruiye has other follow-up products such as cefuroxime, cefodizime and cefpiramide. It is expected that the drug registration approval will be obtained and will be listed one after another.
Source: Minenet database, listed company announcement
Acidity regulators are mainly used to regulate the flavor of foods, and can also serve as preservatives, antioxidants, buffering and other functions.
Our company provide acidity regulators for many food additives, including citric acid, sodium citrate, potassium citrate, lactic acid, malic acid and tartaric acid. In addition to food acidity regulation, can also be used for fine chemical industry, pharmaceutical industry, etc.
Acidity Regulator,Beverage Acidity Regulator,Acidity Regulator Potassium Citrate,High Class Acidity Regulator
Allied Extracts Solutions , https://www.nballiedbiosolutions.com