Introduction and application of iQue Screener ultra high throughput suspension cell/microbead screening system
iQue Screener Ultra High Throughput Suspension Cell/Microbead Screening System Introduction
Shanghai Dian Biotech (Shanghai) Ltd.
High-content screening enables multi-parameter cell experiments to become a routine process in the drug development process, facilitating drug development by providing rich data. However, high-content screening is limited to adherent cells and cannot reach more important research fields. IntelliCyt's screening system, iQue Screener, can provide high-intensity experimental results at the speed required for current screening experiments. Cells and beads. The application of iQue Screener is briefly described as follows:
(1) Increasing the efficiency of small molecule drug development in phenotypic screening experiments by performing high-throughput functional screening of suspended cells;
(2) Better hybridoma information is obtained from antibody development studies by simultaneously obtaining antigen binding and cross-reactivity data, and the screening targets are maintained in their natural configuration state.
(3) Ensure that more in vitro toxicity predictions are obtained through a fully automated, multi-point experimental design that analyzes the health characteristics of thousands of independent cells per second.
Integrated instruments, reagents and software solutions
Our fully integrated screening solutions include instruments, software and kits that allow researchers to analyze suspension cells at the speed and efficiency of screening. IntelliCyt's iQue screening system is a high-volume, flow cytometer-based screening platform that combines high-speed sample delivery with a rich, cell-based data volume. ForeCyt Screening Software provides microplate-centric instrument control, real-time data analysis and the ability to convert screening data into applicable results. IntelliCyt Data Manager (iDM) simplifies data management and enables data sharing between multiple instruments and researchers. MultiCyt Assay Kits are validated, plug-and-play kits that have been optimized for a wide range of cell-based screening applications.
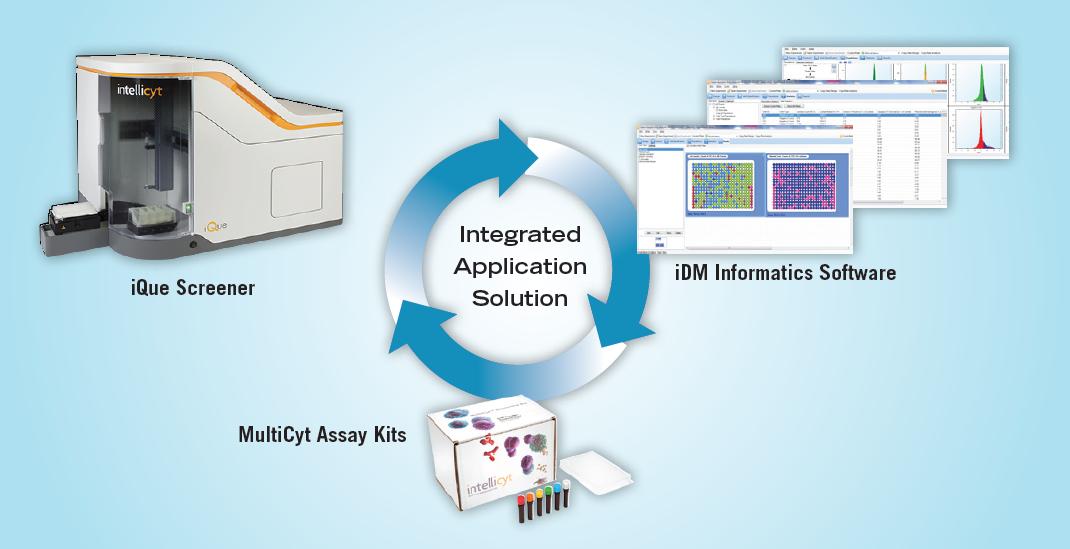
Figure 1. IntellCyt screening platform
How does iQue Screener work?
The iQue screening system delivers a continuous stream of samples to the detector, which collects multiple readings from a single cell. This unique method of injection delivers cells or other inclusions from a microplate to a flow cytometer in a continuous, air-spaced manner to ensure accurate tracking and minimize cross-contamination. The final test results take only a few minutes instead of a few hours, and the microplate-based experimental results can be formed in the form of an easily interpretable heat map, as shown in Figure 2.
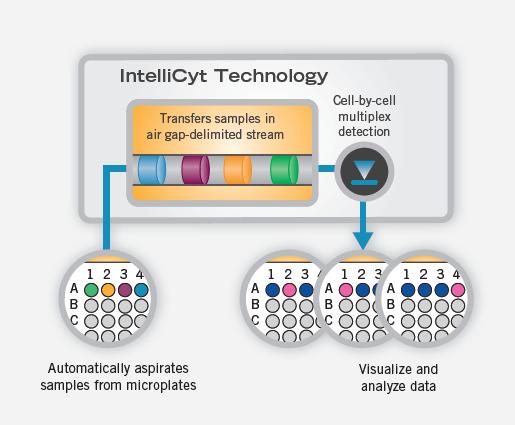
Figure 2. Visualization of injections, tests, and data
The workflow of iQue Screener is as follows: Cells are labeled with MultiCyt Assay Kits or other experimental methods, dispensed in 96-well plates or 384-well plates, and then loaded into the iQue system automatically or manually, in each well. Samples as low as 1 μL are injected into iQue. The instrument performs a single cell test and the data automatically enters the iDM database. Researchers can connect to data on client computers and perform rapid data analysis.
iQue features:
(1) Higher experimental efficiency
(a) Screen your database within a few days, not months;
(b) The injection volume can be as low as 1 μL, reducing the cost of reagents;
(c) Run a 96-well plate in 3 minutes and a 384-well plate in 12 minutes;
(d) can be integrated with the robot arm and other automatic work units;
(e) Compatible cells, microbeads, and mixtures thereof.
(2) More physiological relevance
(a) A mixture of different cells can be analyzed to read data from a single cell;
(b) screening cells in suspension and screening for targets in a natural conformation;
(c) collecting multiple readings using four fluorescent channels;
(d) The size and particle size of the unlabeled target can be detected.
(3) More stable experimental results
(a) The detection range of the 6th power provides sensitive and reliable experimental results;
(b) Detecting up to 10,000 independent cells per second for better statistics in each well;
(4) easier to use
(a) complete workflow support;
(b) Real-time dynamic analysis;
(c) Seamless report generation;
(d) Validated applications and kits.
iQue application introduction:
(1) Antibody Discovery (Antibody Discovery)
Traditional methods of studying antibody properties have been limited to studying a single endpoint, making it difficult to detect multiple cells and multiple conditions. IntelliCyt's screening system, combined with the MultiCyt kit, allows simultaneous detection of multiple endpoints and facilitates high-throughput identification and description of antibodies in cloned libraries. In addition, the use of suspension cells allows the target substances to be screened in their natural configuration to obtain better information on hybridomas.
(1) Hybridoma Screening
Screening hybridoma libraries for antibodies that bind to cell surface antigens is a time-consuming and resource-intensive process because the current screening process involves a series of independent biochemical experimental procedures. In the first step, the combination is detected. The traditional method is to perform an ELISA experiment, which requires purification, separation, and immobilization of the target antigen on the surface of the experimental microplate. Once the conjugate is found at the initial screening, specific experiments are required to test its cross-reactivity with other antigens. Cell-based assays are then used to test whether positive antibodies bind to antigens inside the cell membrane under natural conditions. If the purified protein is a denatured antigen in the above steps, a false negative result will be produced. The combination of IntelliCyt's screening system and the MultiCyt Hybridoma Screening Kit allows for optimization of the entire experimental procedure. This experimental method of mixing and then detecting eliminates many of the existing steps in the primary screening and simultaneously assesses antigen binding and cross-reactivity characteristics (see Figure 3). Because screening can be performed in untreated live cells, using antigens in a natural conformation, conformational epitopes are easily detected, and the likelihood of missing target antibodies or false negatives is greatly reduced (see figure 4).
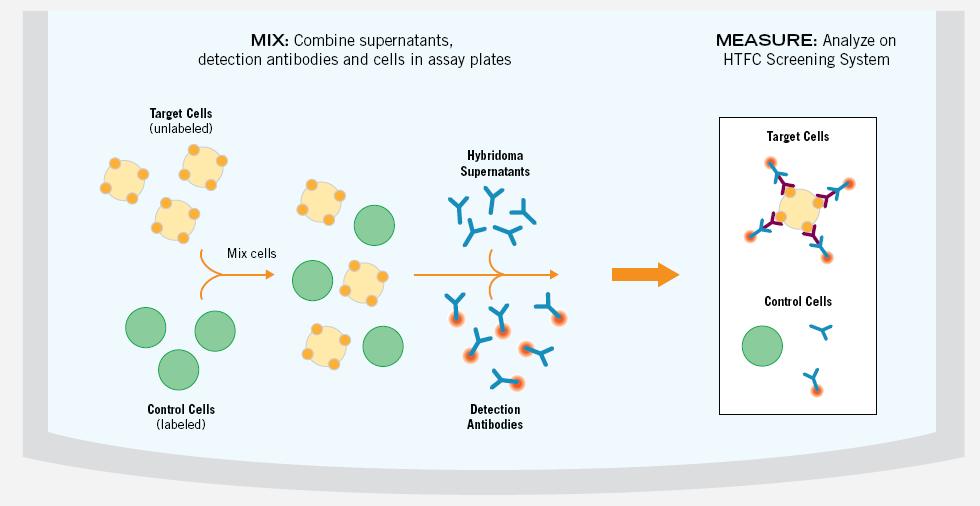
Figure 3. Target cells (unlabeled) and control cells (calcein marker) are mixed together and distributed in a microplate. The test antibody in the hybridoma pool is then added and incubated with the detection antibody with red fluorescence. The microplate was then analyzed with iQue and the binding of antibodies in the hybridoma suspension to the target cell, control cell population was detected. Binding to control cells demonstrated cross-reactivity and non-specific binding, providing an internal control for each well experiment.
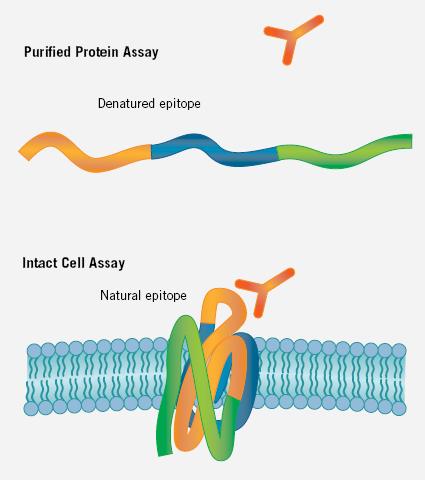
Figure 4. Experiments based on untreated cells allow antibody screening to be performed on conformational epitopes, which is not possible in ELISA and other purified protein experiments. In the latter conditions, antigens are often purified. Lost in the process and lost in the process.
(2) Determine the cross-reactivity between species (Determining Species Cross-Reactivity)
The development and development of antibody therapies involves screening a large number of candidate antibodies, characterizing leading molecules in vitro, and conducting preclinical studies in animal models of efficacy and toxicity. Antibody therapies are unique in that they bind abnormally and specifically to target antigens, but this specificity poses a challenge to downstream research and development processes. For example, a leading candidate antibody that specifically binds to a human target antigen may only bind to the same antigen in other animal germ lines, which may cause difficulties in preclinical studies.
The multiplexing capability of the IntelliCyt screening system incorporates the cross-species reactivity of antibodies into the screening process of development. The MultiCyt Cell Encoder Kit allows for the simultaneous detection of five cell lines per well (see Figure 5). This experiment can easily identify highly suitable clones that cross-react with a variety of germline antigens that are more powerful candidates for preclinical studies. In addition, multi-plate multi-condition experiments can be completed and analyzed in a matter of hours, greatly reducing the time and cost of obtaining large amounts of data.
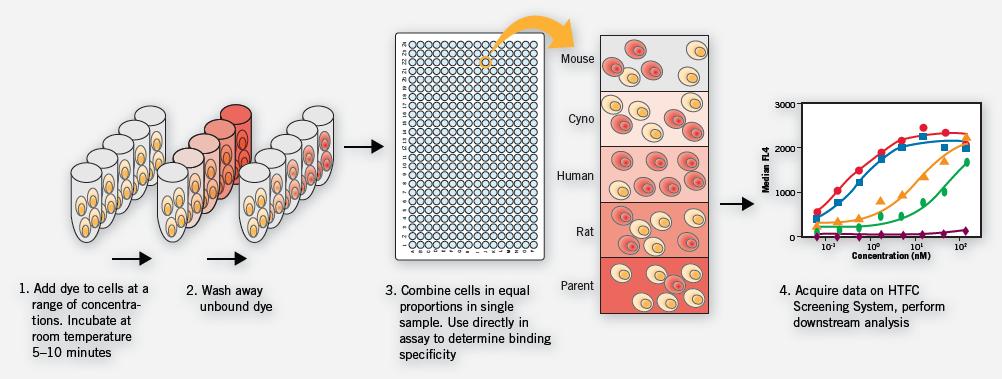
Figure 5. Parental cell lines and genetically engineered cell lines expressing mouse, rat, macaque and human receptors were separately labeled and mixed in one sample. Among them, the untransfected parental cell line served as a negative binding control for each sample. The sample was added to a 96-well microplate for antibody binding experiments, and then the primary screening antibody and the second screening antibody were added in sequence. At the end of the experiment, the cell lines of each sample were confirmed and evaluated separately to generate a binding affinity curve. In this case, experiments were carried out in four 96-well microplates, and 12 antibody responses and 8 dose response curves were obtained under four different experimental conditions, all of which were completed within 1 hour.
(3) Assessing Productivity of Antibody-Secreting Cell Lines
The ability to find highly productive cell lines at an early stage is critical to the success of antibody therapy because optimization and amplification of downstream conditions is time consuming and costly. A common method for assessing the production efficiency of antibody secreted by a cell line involves establishing and culturing a library of clones for production cell lines, followed by high throughput experiments to determine the amount of secreted protein in the supernatant of each clone. However, a typical single endpoint method uses ELISA or other surface interference techniques that do not distinguish between highly productive cell lines and low productivity but fast growing cell lines. For the target selected by this method, a second experiment must be performed to evaluate the amount of antibody secreted per cell.
The combination of the IntelliCyt screening system's multiplexing capability and the Multi-Cyt IgG Count and Capture Kit kits increases the chances of successfully identifying high-productivity cell lines for further downstream analysis. In a single experiment, antibody levels of individual cells can be obtained by simultaneously detecting the amount of antibody secreted, the number of cells, and the activity of the cells (see Figure 6).
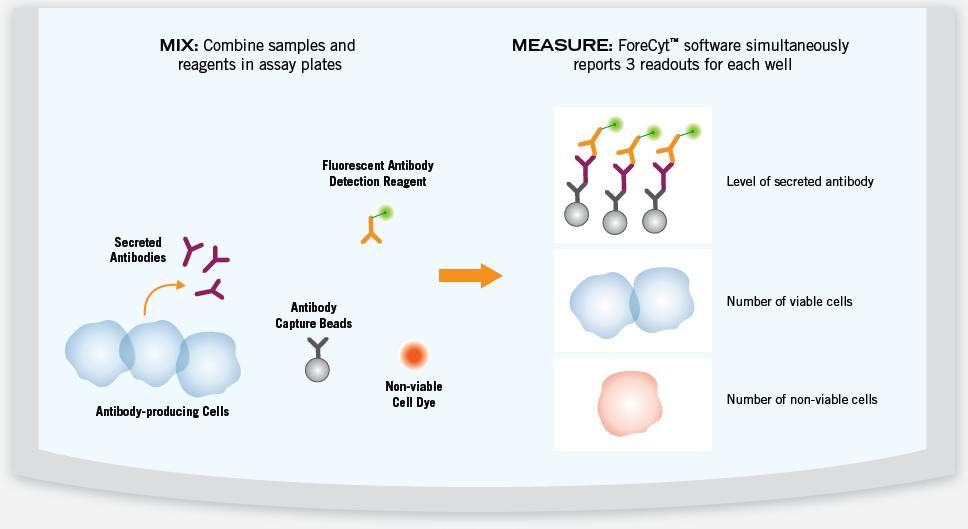
Figure 6. The secreted antibody in the sample binds to the capture beads and is detected by a fluorescently labeled detection reagent. A dye that specifically labels the inactive cells is added to determine the ratio of active cells to inactive cells. The microplates were analyzed and three readings were recorded simultaneously for each sample: the level of secreted antibody, the total number of cells, and the percentage of inactive cells. This makes it easy to calculate the level of antibody secreted by each cell.
(4) Screening a Human Germline IgG Library
Traditional antibody screening methods use purified proteins to produce screening results that satisfy epitope diversity and various functions, but many of the therapeutic targets of interest cannot be purified or immobilized on the surface of the microplate. In addition to the labor and cost of protein purification, experiments such as ELISA may also inactivate antigens, resulting in false negative results - those that can be determined using cell-based assays may be missed, and targets in these experimental methods Antigens exist in their natural conformation. Experiments with multiple readings often require step-by-step testing, even simple multiplexing with controls, which is difficult to perform in many existing modes.
Screening methods can be developed using the IntelliCyt screening system, which is suitable for antigens expressed on the cell surface and can detect multiple endpoints for faster and bioavailable data. Fabrus Inc. (San Diego, Calif.) developed a human germ cell Fab library and performed direct screening of approximately 10,000 IgG-target antigens on mammalian cell surfaces. They used the HTFC screening system for this antibody study to allow target IgG to be screened in the natural environment on the surface of lipid cell membranes. Multiple endpoints and group-based assays make the experiments with built-in transfection controls more robust and reduce the cost of reagents.
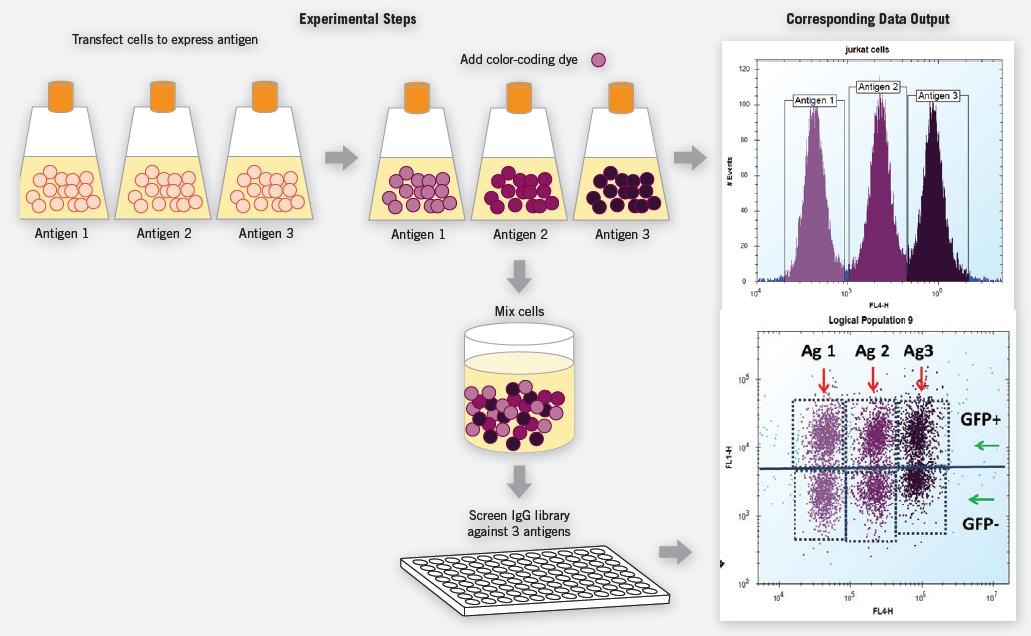
Figure 7. Cells were transiently co-transfected with GFP and the target antigen, and then each group, expressing one of three different target antigens, was labeled with a certain amount of calcein AM violet as the cell-encoding dye. Transfected labeled cells were physically mixed into individual samples and dispensed onto microwell plates for screening of IgG libraries for these samples. The signal of GFP is used to confirm whether the cell expresses the antigen, and the cell-encoding dye enables the three cells to perform experiments simultaneously to confirm and analyze each target. GFP-negative cells served as internal negative controls that did not express the target antigen. The IgG bound to the cells was subjected to a fluorescent secondary, and each stained group was evaluated.
(2) In Vitro Toxicology
If toxicity testing can be performed early in drug development, potential inappropriate results can be identified more quickly and economically. Traditional microplate-based methods for performing high-throughput in vitro toxicity experiments using non-adherent cell lines are challenging because these methods require immobilization of cells on the surface of the microplate. In addition, some test areas are based on manual experiments, so the generation of bio-related results takes a long time, often delaying the follow-up process of drug development. IntelliCyt's screening system analyzes the health of thousands of cells in solution in less than one second, and the fully automated, simplified workflow using MultiCyt kits provides an early screening pathway for genotoxicity for drug development.
(1) Quantification of Micronucleus Induction
Detection of micronuclei (clastogen-induced) and hypodiploid nuclei (aneugen-induced) shows mammalian cells caused by genotoxic compounds Chromosomal damage. A typical approach is to score micronuclei induction using a microscope, but traditional manual methods are both labor intensive and potentially subjective. Automated experiments using high-content imagers can reduce manual time, but the time taken to collect statistically significant data is still long and remains the bottleneck of the experiment. In addition, image-based high content screening is still very time consuming and requires the storage of large amounts of data.
In vitro micronucleus experiments were performed on IntelliCyt's screening system, and Litron (Rochester, New York) in vitro MicroFlow Kit was used to reliably detect micronuclei changes in suspension and adherent cells caused by genotoxic compounds. The labeled cells are lysed, releasing all of the nuclear material, which is analyzed as a separate individual, providing more efficient analysis and more robust experimental signals than image-based methods. When combined with automated equipment and analysis on a 384-well plate, experiments that previously took 16 hours to complete can now be completed in as little as 30 minutes, enabling micronucleus induction experiments to achieve high levels of screening (see Figure 8). In addition, the resulting higher quality data can produce statistically and biologically more relevant screening results and reaction mechanisms, and can significantly reduce the amount of data that needs to be stored.

Figure 8. Experimental setup, incubation, and staining markers as required by the Litron In Vitro MicroFlow Kit. When all staining steps are completed, all nuclear material is released, samples are taken and automated analysis is performed. Complex flow-based gating strategies are applied smoothly and converted into quantitative screening results.
(2) Detection of Apoptotic Events
The process of apoptosis is a highly regulated process of a series of cell morphologies and biochemical events that can be triggered by internal pathways (internal/mitochondrial) or external pathways (external/death receptors). Disorders of this highly regulated event can cause the collapse of normal physiological processes. Therefore, it is important to understand the side effects of candidate drugs on normal apoptosis mechanisms in toxicological screening, or to screen for the development of compounds that modulate the apoptotic process. Because apoptosis is a multi-step, multi-channel process, it is critical that screening experiments can simultaneously assess early or late apoptotic endpoints to obtain more expected results.
IntelliCyt's system, software and application kits are optimized and validated to identify and characterize multiple apoptotic endpoints. Caspase 3, Annexin V, mitochondrial depolarization, cell viability and cell number can be monitored simultaneously or in different combinations to obtain better correlation and completeer Death process map (see Figure 9). Complex cell mixtures can also be monitored to achieve different differential effects.
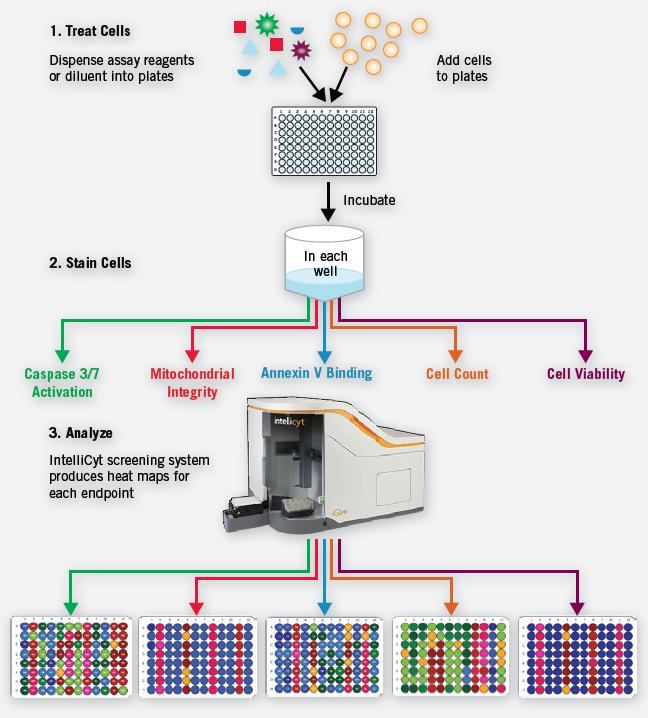
Figure 9. Simultaneous detection of early and late apoptotic endpoints using the MultiMetric Apoptosis Screening Kit. Representative experimental data were obtained from a three-day experiment to detect apoptosis endpoints. Jurkat cells were co-cultured with different concentrations of staurosporine for 24 hours before compound addition. Cells were processed and stained at different time points within three days. EC 50 curves based on 11 points per microplate dilution series (8 replicates) is calculated. The percentage of Caspase 3 positive cells and the percentage of inactive cells were highly consistent in plate-to-plate and day-to-day experiments, with Z' factors at the three endpoints between 0.84 and 0.97.
End point and kit | Apoptotic event |
Caspase 3 Caspase 3 Mix and Read Screening Kit MultiMetric Apoptosis Screening Kit | A key binding site for many apoptotic pathways is the activation of Caspase 3, which ultimately triggers other biochemical and cellular morphological changes. |
Annexin V Annexin V Mix and Read Screening Kit MultiMetric Apoptosis Screening Kit | In the relatively early stages of apoptosis, phosphatidylserine, which normally hides inside the cell membrane, migrates to the surface of the cell membrane. |
Mitochondrial Integrity Mitochondrial Depolarization Mix and Read Screening Kit Annexin V Mix and Read Screening Kit MultiMetric Apoptosis Screening Kit | Depending on the event and path of activation, the mitochondrial membrane will break and release inclusions such as cytochrome C. |
Cell Viability All kits | If severe damage to the cell membrane is detected, this indicates that the cell has entered advanced stages of apoptosis and/or has died. |
Cell Count All kits | The number of cells per sample can be accurately quantified to provide a unique signature of the response characteristics to distinguish between apoptotic events and false positives caused by necrosis. |
Table 1. Apoptosis events were detected using the HTFC screening system and the MultiCyt Kits kit.
(3) Phenotypic Screening
Many specific areas of drug discovery require the study of non-adherent cells, whereas suspension cells are difficult or impossible to study with image-based high-content systems. IntelliCyt's screening system enables multi-parameter measurements of cells that remain in their natural state in suspension. Because cells are read separately, subpopulations can be clearly identified, and cellular artifacts can be rejected to produce more robust, more reliable, and more biologically relevant data. Screening of primary cells or stem cells can also be performed because the system only requires a portion of the cells for traditional flow cytometry experiments.
(1) Screening for Compounds that Induce Myeloid Progenitor Cell Differentiation
Expanding small molecule screening and biomatter screening for known and unknown pathways is a powerful approach to discovering new therapies for the treatment of clinical catastrophic diseases. Using this method, small molecule compounds that manipulate cellular processes such as proliferation and differentiation can be confirmed.
In the case of acute myeloid leukemia (AML), it is very attractive to develop a treatment that can control the arrest of differentiation and trigger the normal differentiation of leukemia stem cells. However, screening for compounds that act on differentiation has been limited in the past by the inability to obtain sufficient model systems. In this example, the HTFC screening system is used to screen for bio-related compounds that overcome the stagnation of differentiation of pro-myeloid progenitor cells, and to readily identify compounds that stimulate stem cell differentiation while eliminating compounds that exhibit cytotoxicity. Because the original suspension cells can now be tested with multiplexed screening, this technology can achieve important achievements in understanding stem cell biology.
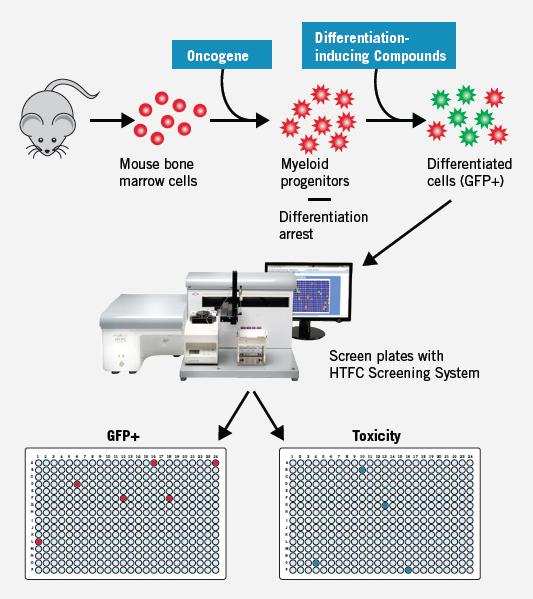
Figure 10. Cell lines were derived from genetically engineered mice expressing GFP under the control of a lysozyme promoter: differentiated cells express GFP (GFP+) and undifferentiated cells do not express GFP (GFP-). At the same time, the cell activity, cell number and green fluorescence of 350,000 compounds were screened. By measuring the forward scatter, side scatter, and number of cells in each well, toxic compounds can be identified without the need for reactive dyes, and GFP+ can be determined simultaneously. Plate heat maps can simultaneously study compounds that induce differentiation (represented by GFP-positive cells) (GFP+ plate), cytotoxicity (represented by loss of cells), and multiple parameters. Ratio (created to set the screening criteria).
(2) GFP Expression Screening in Multiple Cell Lines
The key to developing robust GFP screening experiments is the ability to identify cell populations that efficiently express GFP. This is not possible with PMT-based plate readers and is also very difficult for image-based systems because it is statistically significant from each well of the microplate. The data on the number of sexual cells is very challenging. In addition, the evaluation of other biological endpoints in addition to GFP increases the complexity of most analytical methods.
Compatible with many known GFP models, IntelliCyt's screening system enables phenotypic analysis of high-throughput groups on both suspended and adherent cells. The GFP-expressing cells in the temporarily transfected cell population can be determined and quantified. Multiple GFP signal data at other experimental endpoints such as cell viability can also be detected simultaneously and rapidly. This allows researchers to obtain a more complete and more biologically relevant assessment of high-content screening results.
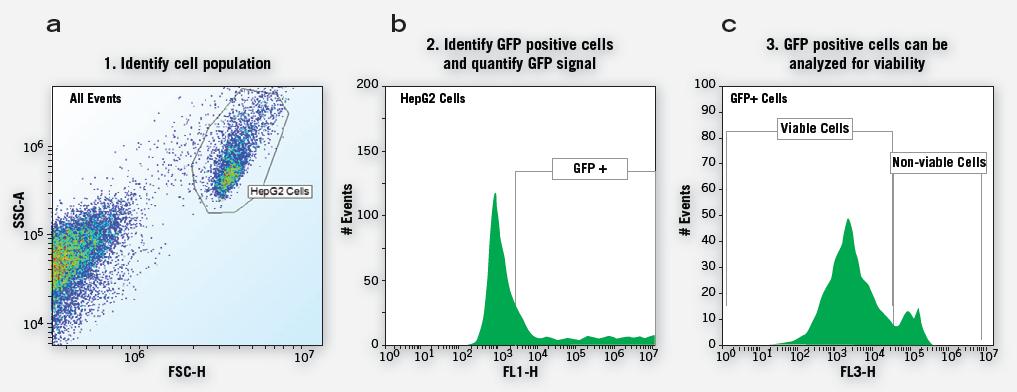
Figure 11. Simplified cohort phenotypic screening process enables multiple endpoints to be analyzed continuously to provide more specific data (eg, GFP signaling from live cells) or as a single endpoint (parallel analysis of cell survival and Percentage of cell expression). Group-based analysis of microplate level data perfectly transforms objective data analysis into filtered statistical data analysis.
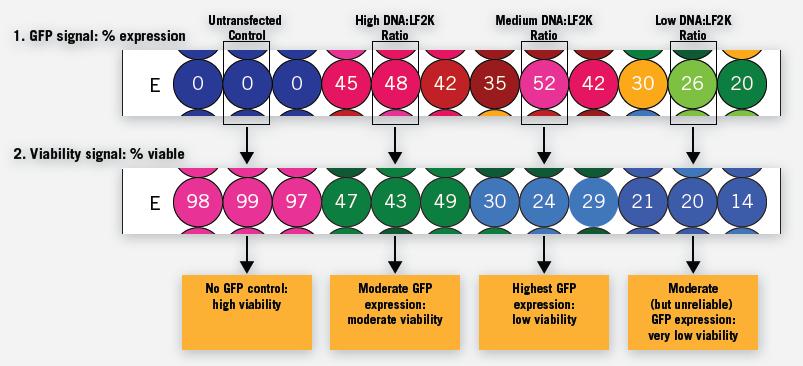
Figure 12. For each sample of the microtiter plate, multiple data can produce more biologically relevant information for data evaluation. Representative data can show different DNA ratios: the triplicate experiment of Lipofectamine (LF2K) transfection reagent on HepG2 cells is shown. GFP expression (expressed as a percentage of expression) per well can be quantified and then cell viability (expressed as percent survival) relative to GFP expression rate can be assessed. For this experiment, the amount of DNA was fixed and the number of LF2K was steadily increased to mediate the ratio of both. Thus, the rightmost low DNA:LF2K ratio can represent a sufficient amount of transfection reagent to induce severe cytotoxicity.
Parameter information:
system message:
Size and weight | 39" W x 24" D x 22" H, 180lb |
Injection | For manual or automatic injection, the autosampler is available for 96-well and 384-well microplates with microplate recall auto setup. In the future, it will also be suitable for 1536-well microplates in existing hardware settings. |
Minimum volume required | The dead volume is zero, the injection volume is 1 μL, and the experimental volume is 10 μL (96-well microplate) |
Screening test method | Flow cytometry |
Detection speed | 10,000 events/second |
Sensitivity range | 6 orders of magnitude (6+ decades) |
Gain and voltage settings | automatic |
Laser excitation | Solid-state lasers: blue (488nm) and red (633nm) |
Multicolor detection | With PMTs, there are 4 simultaneous fluorescence channels |
Unmarked object detection | 2 light scattering channels, detecting object size and granularity |
Sensitivity | Using fixed optical optics, with a dynamic range of 7 orders of magnitude (7 decade) |
Minimum microplate processing speed | The 96-well microplate takes 3 minutes, and the 384-well microplate takes 12 minutes. |
automation | PlateCrane robotic microplate stacker (Hudson Robotics, Inc) and API software (both are optional). Liquid storage tank and overnight operation (both standard) |
Software information:
Enterprise data software | Standard |
Software features | Standardization: dynamic linking gating, interactive heat maps, 1D and 2D plot settings, custom PDF data report, well scan, unified work space, custom metrics, automated cleaning, one data file per plate, templates, advanced screening software. File size per 96-well microplate: 70-100 MB. File output formats: FCS, CSV and ForeCyt plate formats. |
Computer requirements | Windows 7, 64-bit |
Security information
CE certification | by |
For more information, please refer to the IntelliCyt website: and the Codex website:
Shanghai Dian Biotech Co., Ltd. Contact: Room 2101, Building 6, Huiyang Building, 1139 Pudong Avenue, Shanghai. Tel: (+86) 21-58605185-807 (Miss Liu), fax: (+86) 21-51973282, E-mail: and.
Unlike fresh fruits, fruit powder is made by advanced technologies so to extend the shelf life and versatility. We have Freeze Dried Fruit Powder and spray dried fruit powder. The star freeze dried powder are strawberry powder, raspberry powder, pink pitaya powder, blueberry powder and banana powder. Our fruit powder have no added sugar, sweeteners, preservatives,colors or flavorings which makes it perfect additive to food and beverage like ice cream, smoothie and sauce. Fruit powder promote bowel function and clean toxins, lower cholesterol absorption.
Fruit Powder,Mulberry Powder,Banana Powder,Apple Powder
YT(Xi'an) Biochem Co., Ltd. , https://www.ytnutra.com