Entering solid tumors, alleviating CRS, and looking forward to new developments in cellular immunotherapy
Cancer occurs when most of the cells in the human body begin to undergo uncontrolled division. Cancers such as cell division and differentiation have emerged from ancient times, and with the advancement of science and technology, people have stepped into the microscopic world and began to look for ways to cure such diseases from the perspective of cellular molecules.
At present, the most favored technology is cell immunotherapy. People try to eliminate cancer from the perspective of cells, and to eliminate the certain diseases from the "eyes of fire" that are specific to different types of immune cells. CAR-T therapy is the most recognized and fastest-growing cell therapy, and two products have been approved for marketing (Kymriah and Yescarta).
The cell therapy team is certainly far more than the CAR-T member, and cell therapy such as NK and TCR-T is also promising. The authors collated some of the new trends in recent cell therapies to witness the advances in cell therapy.
The world's first "CAR-T treatment of AIDS" patent was awarded to the two professors of China Wuke University
On November 27th, Professor Zhang Tongcun and Gu Chaojiang from the School of Life Science and Health of Wuhan University of Science and Technology obtained the construction of a recombinant gene for Chimeric Antigen Receptor (CAR, Editor's Note). Its application" invention patent certificate. This is the world's first application of CAR-T cell therapy to treat AIDS, and will provide new treatment ideas for the cure of AIDS.

Image source: Wuhan University of Science and Technology official website
Professor Zhang Tongcun has been specializing in the CAR-T field for many years and has rich CAR-T clinical experience. He is the “father of promotion†of China's CAR-T, and Gu Chaojiang teaches more than ten years of research experience in AIDS. In 2015, each of them took the lead and worked together to create a new treatment for “using CAR-T technology to treat AIDSâ€.
AIDS is an immunodeficiency disease. After the HIV virus enters the human body, it attacks the human immune system. After the immune system is destroyed, the human body cannot resist other external infections and die, and the CAR-T technology is simply Teach immune T cells to recognize specific antigens and target attack pathogens.
In this study, a broad-spectrum neutralizing antibody with highly specific binding of viral proteins gp120 and gp41 was used as a scFv (single-chain variable fragment), which binds to most HIV viruses. The scFv fusion protein gene "church" T cells specifically recognize and destroy HIV-infected cells and neutralize HIV in the blood.
At present, the human clinical trials conducted through this technology are also effective. Among the two HIV patients, one patient has a significant reduction in HIV virus within three months, and the other two patients are completely clear after nine months of treatment. HIV virus. In addition, this technology can eliminate the sleeping cells in dormancy and put an end to the return of HIV.
FDA approves Fate NK pipeline IND application
On November 30th, Fate Therapeutics announced that the FDA has approved its IND application for FT500. In the subsequent American Society of Hematology (ASH), they announced the recent FT500 and immune checkpoint PD-1 drugs, immune T Clinical data on the combination of cells showed that 99% of tumors have shrunk.
Fate Therapeutics is a US biotechnology company that develops stem cell therapies using basic biological mechanisms. Founded in 2007, it has completed nine rounds of financing, totaling $160 million. The company has two major NK cell lines, one is a strong killing NK cell from healthy human peripheral blood culture, and the other is a high affinity NK cell differentiated from induced pluripotent stem cells (iPSC). The approved FT500 is a natural killer (NK) cell that induces pluripotent stem cells (iPSC).
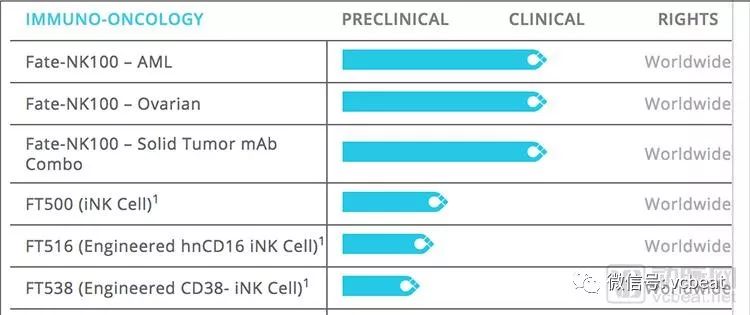
Fate Therapeutics part of the research and development pipeline
NK cells, the natural killer, are the front-line cells of the immune system and belong to the innate immune system, which has a broad-spectrum anti-tumor effect. FT500 can provide a large number of NK cell populations, and the stress ligands on the exposed tumors release cytotoxic granules, which can directly lyse the tumor. At the same time, the cytokines secreted by NK cells can enhance the activity of T cells and recognize the tumor cells under the cooperation of T cells. Make it wipe out. In addition, the FT500 can be cryopreserved for clinical repeated administration.
Mr. Scott Wolchko, CEO of Fate Therapeutics, said, “This time, the FDA approved the IND application for the FT500, an important milestone that marks the beginning of a new era in the clinical development of cellular products.â€
Inhibition of glutamine metabolism enhances CAR-T efficacy
On November 1st, the biological research team led by Dr. Jeffrey Rathmell from Vanderbilt University published a paper in Cell, saying that after inhibiting the metabolism of an amino acid called glutamine, it regulates cancer. The reacting T cells become active and enhance the function of CAR-T in cell therapy.

Title of the paper "Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism"
Glutamine is an essential amino acid for many cells in the body to maintain normal function, while abnormally dividing cancer cells have more demand for glutamine. The original experiment was to "starve" cancer cells by inhibiting the metabolism of glutamine. This will inevitably hurt the innocent, and let the T cells follow the "starving" - reducing the activity.
However, the results of the study were diametrically opposite. After knocking out the glutaminase-encoding gene in mice, it was unexpectedly found that some T cells in the mice became abnormally active, and this part of the active T cells was involved in anti-cancer and anti-viral reactions. T cells.
Professor Jeffrey Rathmell also responded by saying that in short, some T cells require glutamine, while others do not. The T cells required for eliciting autoimmunity, for example, are less active, and the anti-cancer T cell activity that is not required is increased. After further testing of the mouse CAR-T model, it was also found that in mice that inhibited glutamine metabolism, CAR-T cells not only increased in time but also enhanced in function, but functional enhancement was only short-term.
In this experiment, inhibition of glutamine metabolism is a CD-839 glutaminase inhibitor, and the experimental team also plans to use it in combination with the immunological checkpoint PD-1 inhibitor Opdivo to test various dosing regimens.
Our mission: To protect the health of ear, throat and nose with medical frontier knowledge and technological innovation.
Our vision: Leader in several niche markets of Otolaryngology.
Our values: Change, Enterprising, Share
Silicone Enema Bag, Enema Bulb, Enema Bucket, Automatic Enema Device
Ningbo Jiamai Internet Technology CO., Ltd. , https://www.jmcuhyd.com