Foreword
The analysis of active pharmaceutical ingredients (API) and finished drug impurities is an integral part of drug development. The chemical structure information of impurities is critical in assessing toxicity, improving synthetic pathways, and selecting the optimal dosage form based on the target drug.
To ensure drug efficacy and consumer safety, regulatory agencies around the world have developed guidelines for the analysis of drug impurities, clearly indicating the limits based on daily dose, time of use, and drug targets.
LCMS has become the most commonly used unknown impurity analysis technique. In order to be able to identify and characterize trace impurities in a large amount of parent drug, mass spectrometers with high sensitivity, high scanning speed, and multi-stage mass spectrometry (MSn) are essential.
In this study, research-grade adefovir dipivoxil (ADP) sold by Sigma-Aldrich was used as a model compound for API impurity analysis. ADP is an antiviral prescription drug widely used for chronic hepatitis B virus infection. The LCMS system used in this study consisted of a Thermo Scientific AccelaTM UHPLC and a Velos ProTM linear ion trap mass spectrometer. Fragmentation information is obtained by combining CID and Trap-HCD dissociation techniques. Data processing is done through the software Mass FrontierTM.
Analytical method
Materials and reagents
Adefovir dipivoxil (CAS # 142340-99-6) was purchased from Sigma-Aldrich under the product number A9730.
Acetonitrile and water (Fisher); ammonium acetate (Sigma-Aldrich, product number 73594-25G-F); formic acid (Sigma-Aldrich, product number 33015-500 ml).
Sample Preparation
ADP solution, 0.5 mg/mL, was obtained by dissolving 0.5 mg of ADP in 1 ml of a 20:80 mixed solvent of acetonitrile and water.
HPLC method
HPLC system: Thermo Accela 1250 pump, open Accela autosampler and Accela PDA
Column: Hypersil GOLD C18 2.1 x 150 mm, 3 μM,
Thermo Scientific Product Number 071399
Column temperature: 35 °C
Flow rate: 0.5 ml / min
Injection volume: 8 μl
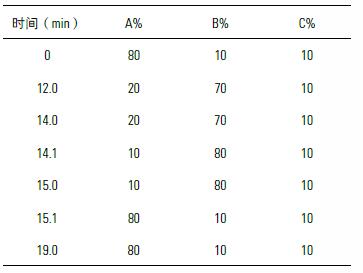
Mobile phase: A – water, B – acetonitrile, C - 100 mM ammonium acetate, adjusted to pH 5 with acetic acid
Gradient elution:
Mass spectrometry
Mass spectrometer: Velos ProTM linear ion trap ionization conditions with HESI-II ion source: electrospray, positive ion mode
1. Full scan mode m/z 120-1000 amu/ Top5 dd MS/MS, HCD NCE 35%
2. Full scan mode / by parent ion list dd MS2/ TOP 3 ddMS3, CID/ Trap-HCD NCE 20%
a. CID MS 2 / CID MS 3
b. CID MS 2 / HCD MS 3
c. HCD MS 2 / CID MS 3
d. HCD MS 2 / HCD MS 3
Results and discussion
I. Full Scan - Top5 HCD dd MS/MS and " FISh "
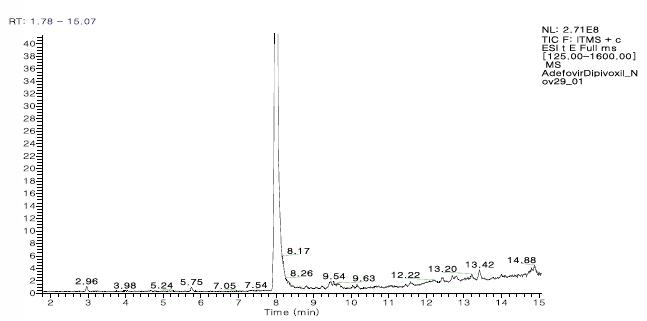
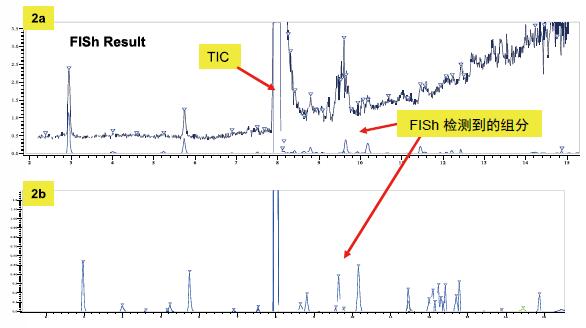
The ADP impurity profile analysis process consisted of two consecutive LCMS experiments. The goal of the analysis is to collect as much information as possible for impurity identification and structural analysis. The first MS experiment included full scan and subsequent Top5 HCD data depending on the MS/MS scan (Figure 1). Next, the data is processed by the Fragment Ion Search algorithm in MassFrontier, which is a novel method for impurity detection and identification, based on the comparison of the parent ion dissociation modes (Fig. 2a, b). ). The ingredients with the same fragments as the parent drug are identified by "FISh", see Figure 3.
Figure 1. Mass spectrometry total ion chromatogram of adefovir dipivoxil
Figure 2. Detection of impurity components using the Mass Frontier "FISh" algorithm
Label: The components detected by FISh
Figure 3. XIC of impurity components detected by the "FISh" algorithm
II.Trap-HCD/CID MS 2 And MS 3 provides rich fragmentation information for structural analysis of unknown compounds
Further structural information was obtained by combining the parent compound list with the Trap-HCD and CID dissociation combination for MS 2 experiments.
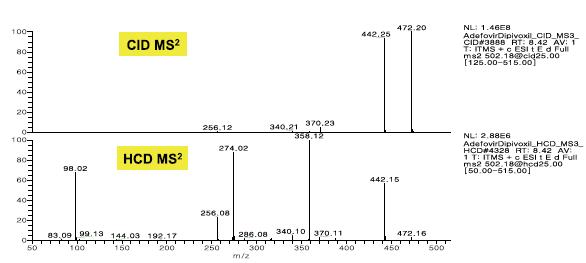
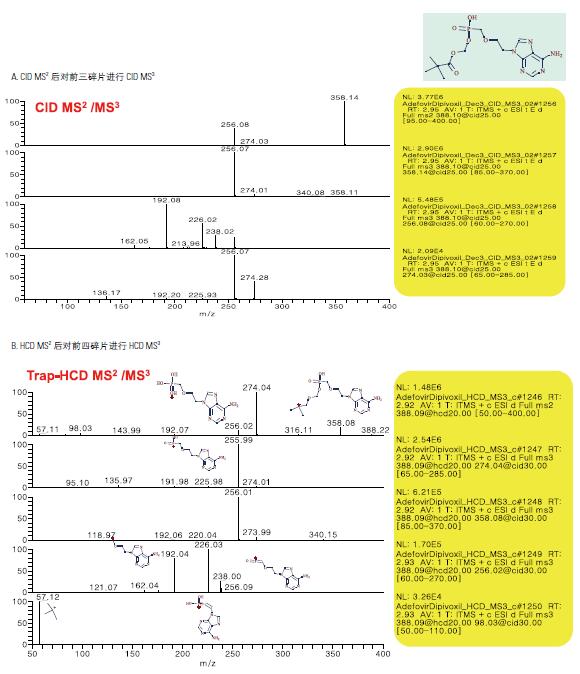
When the normalized collision energy levels are the same, Trap-HCD is richer than the fragmentation information generated by CID. Trap-HCD has no low-quality discriminatory effects, as shown in Figure 4. CID MSn provides step-by-step cracking information and is very valuable for structural analysis, see Figure 5, 6.
Figure 4. CID & HCD MS 2 spectra of adefovir dipivoxil at the same 25% NCE level
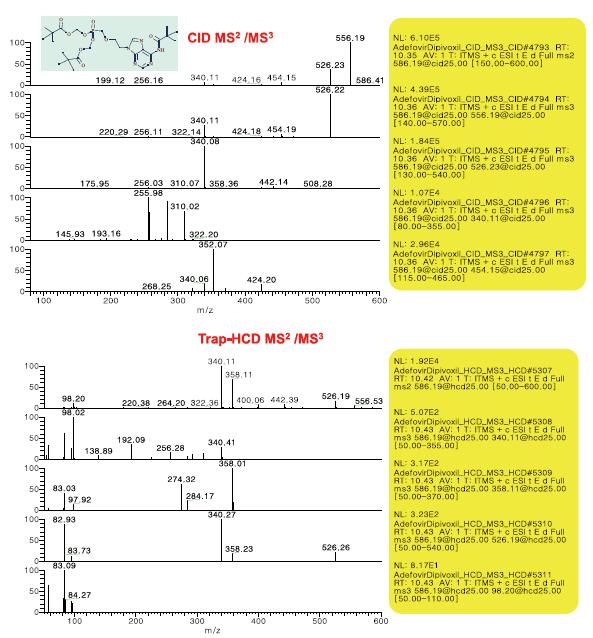
- Full sweep, CID dd MS 2 (set parent ion list), then HCD MS 3 for the first three MS 2 fragment ions
- Full sweep, CID dd MS 2 (set parent ion list), then CID MS 3 for the first three MS 2 fragment ions
- Full sweep, HCD dd MS 2 (set parent ion list), then CID MS 3 for the first three MS 2 fragment ions
- Full sweep, HCD dd MS 2 (set parent ion list), then HCD MS 3 for the first three MS 2 fragment ions
Figure 5. Comparison of CID and HCD spectra of impurity m/z 558.19
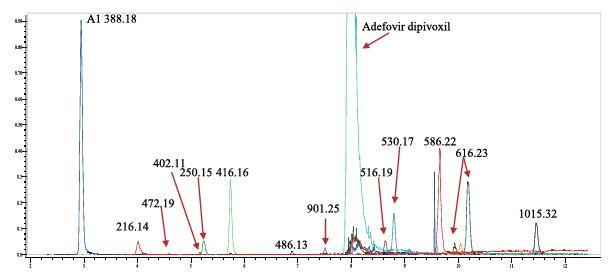
Figure 6. CID and HCD MS 2 , MS 3 spectra of impurity m/z 388.13
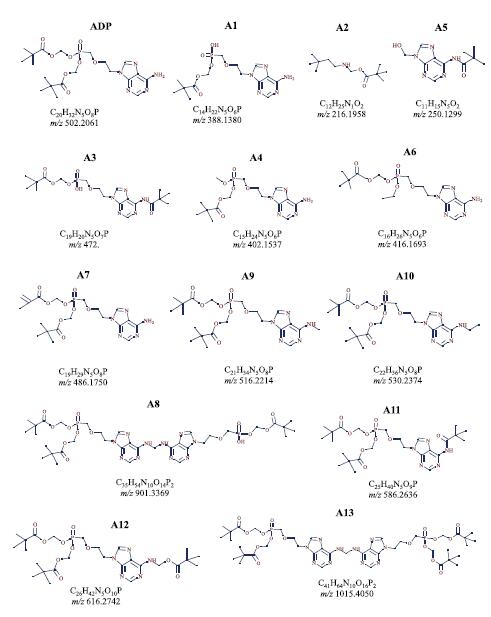
Figure 7. Possible structural formula of impurities contained in a sample of research-grade adefovir dipivoxil sold by Sigma-Aldrich.
Based on the rich HCD, CID MS 2 and MS 3 fragmentation information (see Figure 7), the identified impurity compounds were structurally predicted. The exact mass and elemental composition of each impurity component was confirmed by high resolution data from a benchtop Orbitrap Q ExactiveTM mass spectrometer.
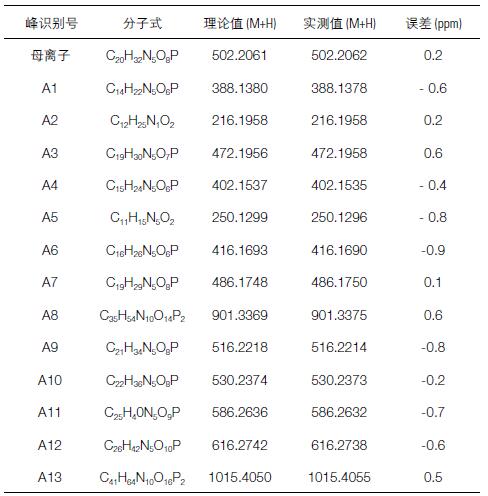
Table 1. Accurate mass and elemental composition of impurity components confirmed by Q Exactive high resolution data
in conclusion
Two dissociation techniques—Trap-HCD and CID—are used to characterize thirteen low-level impurities in commercially available research-grade adefovir dipivoxil samples. When the normalized collision energy levels are the same, Trap-HCD is richer than the fragmentation information generated by CID. Trap-HCD does not have a low quality discrimination effect. On the other hand, CID can provide step-by-step cleavage information and MS n spectra, which is very valuable for clarifying substructure associations. The results of this study demonstrate that combined with the data post-processing software using these two complementary dissociation methods can excellently complete impurity identification and structural analysis, and improve the speed and reliability of unknown impurity analysis.
Related Links
Drug Impurity Analysis Solution--Ion Trap Multi-Level Liquid Quality
Fruit powder
Apple Powder is a kind of Apple extract and a Fruit&Vegetable Power of our company. It is a light yellow
Powder , concentrated and dried from Apple fruits.
Apple is the apple of the apple subfamily of the Rose family, the tree is deciduous trees.Apple nutritional value is very high, rich in minerals and vitamins, rich in calcium, help to metabolize excess salt in the body, malic acid can metabolize
heat, prevent obesity in the lower body.Apple is a kind of food with low quantity of heat, produce the quantity of heat of 60 kilocalorie or so every 100 grams.
There are hundreds of apple varieties, which are divided into three categories: wine varieties, cooking varieties and fresh food varieties.The three varieties differ in size, color, aroma, smoothness (and perhaps brittleness, flavor) and other characteristics.Many varieties are high in sugar, moderate in acidity and low in tannin.In addition to being eaten raw, apples can be prepared in a variety of ways. They are often used as pastry fillings. Apple scones are probably the first American sweet.Fried apple often with sausage, pork chops and other dishes with food, especially in Europe is particularly common.The latest taxonomic evidence suggests there are just 38 species in the genus Apple.
Apple Juice Powder,Apple Juice Powder Mix,Chitosan Apple Juice Powder,Green Apple Juice Powder
Shaanxi Kepler Biotech Co.,Ltd , https://www.keplerherb.com